Part 2: Quantum theory of light
... Shortly after J.J. Thompson's experiments led to the identification of the elementary charged particles we now know as electrons, it was discovered that the illumination of a metallic surface by light can cause electrons to be emitted from the surface. This phenomenon, the photoelectric effect, is s ...
... Shortly after J.J. Thompson's experiments led to the identification of the elementary charged particles we now know as electrons, it was discovered that the illumination of a metallic surface by light can cause electrons to be emitted from the surface. This phenomenon, the photoelectric effect, is s ...
PHYS 221: Homework Assignment 3 This homework due just prior
... a) [6 points] What is the greatest possible value that the wavelength λ of the electron could have and still be consistent with these facts? [Give your answer in terms of the given fixed quantities a and θ] b) [2 points] Now suppose that the electron is replaced by a photon having the same wavelengt ...
... a) [6 points] What is the greatest possible value that the wavelength λ of the electron could have and still be consistent with these facts? [Give your answer in terms of the given fixed quantities a and θ] b) [2 points] Now suppose that the electron is replaced by a photon having the same wavelengt ...
E ref (W)
... Average depth (mean range) of atomic carbons implanted in crystalline tungsten at normal incidence on (001) tungsten surface and amorphous cell surface as a function of incident energy. The results calculated by Eckstein using TRIM.SP code are also shown. ...
... Average depth (mean range) of atomic carbons implanted in crystalline tungsten at normal incidence on (001) tungsten surface and amorphous cell surface as a function of incident energy. The results calculated by Eckstein using TRIM.SP code are also shown. ...
AP Unit 1 Test Review
... (D) Iodine liberates free bromine from a solution of bromide ion. (E) Fluorine is the most electronegative of the halogens. 8. Question 8-11 refer to atoms for which the occupied atomic orbitals shown below. 8. Represents an atom that is chemically unreactive 9. Represents an atom in an excited stat ...
... (D) Iodine liberates free bromine from a solution of bromide ion. (E) Fluorine is the most electronegative of the halogens. 8. Question 8-11 refer to atoms for which the occupied atomic orbitals shown below. 8. Represents an atom that is chemically unreactive 9. Represents an atom in an excited stat ...
Science, Systems, Matter, and Energy
... – Lack carbon-carbon or carbonhydrogen covalent bonds – NaCl, H2O, N2O, CO2, NH3 ...
... – Lack carbon-carbon or carbonhydrogen covalent bonds – NaCl, H2O, N2O, CO2, NH3 ...
Chapter 2: You must understand chemistry to understand life (and to
... 5. elements differ from each other because they contain different numbers of protons (all hydrogen atoms contain 1 proton, all carbon atoms contain 6 protons, all oxygen atoms contain 8 protons, etc.) atomic number = number of protons in the nucleus the periodic table has elements arranged large ...
... 5. elements differ from each other because they contain different numbers of protons (all hydrogen atoms contain 1 proton, all carbon atoms contain 6 protons, all oxygen atoms contain 8 protons, etc.) atomic number = number of protons in the nucleus the periodic table has elements arranged large ...
Quantum Number, n. - Lyndhurst Schools
... When light of a sufficiently high energy strikes a metal surface, electrons are knocked off its surface. • Einstein assumed that light traveled in energy packets called photons. • The energy of one photon is: ...
... When light of a sufficiently high energy strikes a metal surface, electrons are knocked off its surface. • Einstein assumed that light traveled in energy packets called photons. • The energy of one photon is: ...
Chapter 6: Electronic Structure of Atoms
... The negative sign indicates that this frequency light is emitted when an electron goes from n = 5 to n = 2. (If an electron were to go from n = 2 to n = 5, light of the same frequency would be absorbed.) This corresponds to the 434.8-nm line in the hydrogen line spectrum. Thus, energy must be absorb ...
... The negative sign indicates that this frequency light is emitted when an electron goes from n = 5 to n = 2. (If an electron were to go from n = 2 to n = 5, light of the same frequency would be absorbed.) This corresponds to the 434.8-nm line in the hydrogen line spectrum. Thus, energy must be absorb ...
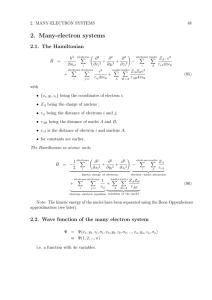
Links between the Einstein`s Special Relativity DS and
... And so Ek = (γ – 1)moc² = 0.061moc² = 0.061 x 9.1x10-31 x 9x1016 = 5.0 x 10-15 J. This is a little more than the 4 x 10-15 J we calculated above for the obvious reason: In order for the electron in the TV tube to reach ⅓c it will require more than 4 x 10-15 J because, as we know from relativity, it ...
... And so Ek = (γ – 1)moc² = 0.061moc² = 0.061 x 9.1x10-31 x 9x1016 = 5.0 x 10-15 J. This is a little more than the 4 x 10-15 J we calculated above for the obvious reason: In order for the electron in the TV tube to reach ⅓c it will require more than 4 x 10-15 J because, as we know from relativity, it ...
Atomic quantum and nuclear
... masses. For the same reason, remembering that KE = p2/2m, they cannot have the same kinetic energy. Because the kinetic energy is the only type of energy an isolated particle can have, and we have argued that the particles have different energies, Equation 27.15 tells us that the particles do not ha ...
... masses. For the same reason, remembering that KE = p2/2m, they cannot have the same kinetic energy. Because the kinetic energy is the only type of energy an isolated particle can have, and we have argued that the particles have different energies, Equation 27.15 tells us that the particles do not ha ...
Chemical Reaction
... –Exothermic reactions require a smaller amount of activation energy than endothermic reactions ...
... –Exothermic reactions require a smaller amount of activation energy than endothermic reactions ...
Chemistry for Changing Times 11th Edition Hill and Kolb
... Electron Arrangement: The Bohr Model When electrons are in the lowest energy state, they are said to be in the ground state. When a flame or other source of energy is absorbed by the electrons, they are promoted to a higher energy state (excited state). When an electron in an excited state returns t ...
... Electron Arrangement: The Bohr Model When electrons are in the lowest energy state, they are said to be in the ground state. When a flame or other source of energy is absorbed by the electrons, they are promoted to a higher energy state (excited state). When an electron in an excited state returns t ...
國立屏東教育大學95學年度研究所碩士班入學考試
... (A) must be rigid and have rough surfaces (B) must be rigid and chemically inert (C) must be rigid and must not degrade over time (D) must be flexible and have an open porous structure (E) should be designed such that it encourages coagulation of blood 第 1 頁,共 5 頁 ...
... (A) must be rigid and have rough surfaces (B) must be rigid and chemically inert (C) must be rigid and must not degrade over time (D) must be flexible and have an open porous structure (E) should be designed such that it encourages coagulation of blood 第 1 頁,共 5 頁 ...
HOMEWORK ASSIGNMENT 12
... applied along the x-axis. Find an approximation for the low-lying energy levels that is valid in the limit qr0 E0 ~2 /M r02 . Hint: try expanding around the potential about a stable equilibrium point. Here we need to add the electric monopole energy. The electrostatic potential of a uniform E-fiel ...
... applied along the x-axis. Find an approximation for the low-lying energy levels that is valid in the limit qr0 E0 ~2 /M r02 . Hint: try expanding around the potential about a stable equilibrium point. Here we need to add the electric monopole energy. The electrostatic potential of a uniform E-fiel ...
X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition at the parts per thousand range, empirical formula, chemical state and electronic state of the elements that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 0 to 10 nm of the material being analyzed. XPS requires high vacuum (P ~ 10−8 millibar) or ultra-high vacuum (UHV; P < 10−9 millibar) conditions, although a current area of development is ambient-pressure XPS, in which samples are analyzed at pressures of a few tens of millibar.XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its as-received state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some or all of the surface contamination (with mild ion etching) or to intentionally expose deeper layers of the sample (with more extensive ion etching) in depth-profiling XPS, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.XPS is also known as ESCA (Electron Spectroscopy for Chemical Analysis), an abbreviation introduced by Kai Siegbahn's research group to emphasize the chemical (rather than merely elemental) information that the technique provides.In principle XPS detects all elements. In practice, using typical laboratory-scale X-ray sources, XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot easily detect hydrogen (Z = 1) or helium (Z = 2).Detection limits for most of the elements (on a modern instrument) are in the parts per thousand range. Detection limits of parts per million (ppm) are possible, but require special conditions: concentration at top surface or very long collection time (overnight).XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion-modified materials and many others.XPS is less routinely used to analyze the hydrated forms of some of the above materials by freezing the samples in their hydrated state in an ultra pure environment, and allowing or causing multilayers of ice to sublime away prior to analysis. Such hydrated XPS analysis allows hydrated sample structures, which may be different from vacuum-dehydrated sample structures, to be studied in their more relevant as-used hydrated structure. Many bio-materials such as hydrogels are examples of such samples.