+ H 2 O(g)
... Info on Decomp Reactions • Energy is usually need to make these reactions happen • Often hard to predict products unless the substance breaks into its ...
... Info on Decomp Reactions • Energy is usually need to make these reactions happen • Often hard to predict products unless the substance breaks into its ...
Chemical Reactions Unit Pupils` Learning Outcomes
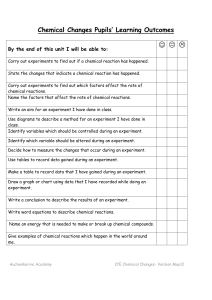
... Identify variables which should be controlled during an experiment. Identify which variable should be altered during an experiment. Decide how to measure the changes that occur during an experiment. Use tables to record data gained during an experiment. Make a table to record data that I have gained ...
... Identify variables which should be controlled during an experiment. Identify which variable should be altered during an experiment. Decide how to measure the changes that occur during an experiment. Use tables to record data gained during an experiment. Make a table to record data that I have gained ...
CHEMICAL REACTIONS
... ________ 20. The complete combustion of octane (C8H18) would: a. require 25 O2(g). c. produce 18 H2O(g). b. produce 16 CO2(g). d. all of the above ________ 21. Double-replacement reactions are generally driven by the formation of: a. a precipitate. c. water. b. a gaseous product. d. all of the above ...
... ________ 20. The complete combustion of octane (C8H18) would: a. require 25 O2(g). c. produce 18 H2O(g). b. produce 16 CO2(g). d. all of the above ________ 21. Double-replacement reactions are generally driven by the formation of: a. a precipitate. c. water. b. a gaseous product. d. all of the above ...
Chapter 6
... which an acid reacts with a base to yield water plus an ionic compound called a salt. – The driving force of this reaction is the formation of the stable water molecule. ...
... which an acid reacts with a base to yield water plus an ionic compound called a salt. – The driving force of this reaction is the formation of the stable water molecule. ...
Chem vocab quiz definitons
... Matter anything that has volume and mass. Proton is a positively charged particles that are found in the nucleus, and has the mass of 1 atomic unit (amu). Neutron is an electrically neutral particles that are found in the nucleus, and has a mass of 1 amu. Electron is a negatively charged sub atomic ...
... Matter anything that has volume and mass. Proton is a positively charged particles that are found in the nucleus, and has the mass of 1 atomic unit (amu). Neutron is an electrically neutral particles that are found in the nucleus, and has a mass of 1 amu. Electron is a negatively charged sub atomic ...
syllabus for entrance examination - NTU.edu
... and m are both integral and are either 0, 1 or 2. The use of the integrated forms of first- and second-order rate equations is not required but the use of constancy of half-life as a test for first order kinetics is included. Simple calculations on half-life may be set. Questions will not be set req ...
... and m are both integral and are either 0, 1 or 2. The use of the integrated forms of first- and second-order rate equations is not required but the use of constancy of half-life as a test for first order kinetics is included. Simple calculations on half-life may be set. Questions will not be set req ...
Chemical Reactions
... the reaction without itself being used up in the reaction (doesn’t appear as a reactant or a product) Catalysts lower the activation energy required for a reaction to occur. Thus a catalyst creates a different pathway from reactants to products – one that requires less energy. Catalysts in the ...
... the reaction without itself being used up in the reaction (doesn’t appear as a reactant or a product) Catalysts lower the activation energy required for a reaction to occur. Thus a catalyst creates a different pathway from reactants to products – one that requires less energy. Catalysts in the ...
File
... 16. What are enantiomers? How can they be identified? 17. What are the micro-alloys? Explain with two examples. 18. Half-life period of a radioactive element is 100 seconds. Calculate the disintegration constant and average life period. How much time will it take for 90% decay? 19. (a) Describe the ...
... 16. What are enantiomers? How can they be identified? 17. What are the micro-alloys? Explain with two examples. 18. Half-life period of a radioactive element is 100 seconds. Calculate the disintegration constant and average life period. How much time will it take for 90% decay? 19. (a) Describe the ...
Chemistry 21 A - El Camino College
... 5. Combustion belongs to _________________________ type of reactions. 6. Rusting of iron is (circle the correct answers – there may be more than one correct answer): a) decomposition reaction b) combustion reaction c) single replacement reaction d) nonredox reaction e) redox reaction f) combination ...
... 5. Combustion belongs to _________________________ type of reactions. 6. Rusting of iron is (circle the correct answers – there may be more than one correct answer): a) decomposition reaction b) combustion reaction c) single replacement reaction d) nonredox reaction e) redox reaction f) combination ...
Erik`s Chemistry: Thermochemistry - ECHS Chemistry
... (James Joule, 1818-1889, Joule also developed the First Law of Thermodynamics): energy is neither created nor destroyed in ordinary chemical or physical changes. b. Quantitative H H = qreaction mixture (at constant temperature only) q = (m)( t)(Cp) q = heat absorbed by the water in joules (J) m = ma ...
... (James Joule, 1818-1889, Joule also developed the First Law of Thermodynamics): energy is neither created nor destroyed in ordinary chemical or physical changes. b. Quantitative H H = qreaction mixture (at constant temperature only) q = (m)( t)(Cp) q = heat absorbed by the water in joules (J) m = ma ...
CHEMISTRY
... A process by which one or more substances are changed into one or more different substances Reactants – the original substances Products – the resulting substances Mass is always conserved ...
... A process by which one or more substances are changed into one or more different substances Reactants – the original substances Products – the resulting substances Mass is always conserved ...
Chemistry 2nd Semester Final Exam Review Chemical Bonds Give
... 19. If 735 L of a gas is at 3.11 atm and 34 oC, what is its temperature at 6.11 atm and 235 L? 20. If 12.2 mL of a gas is at 178oC, what is its volume at 53.0oC? 21. What are the values of STP? What is the meaning behind STP? 22. What is the lowest temperature possible? What is it called? Acids and ...
... 19. If 735 L of a gas is at 3.11 atm and 34 oC, what is its temperature at 6.11 atm and 235 L? 20. If 12.2 mL of a gas is at 178oC, what is its volume at 53.0oC? 21. What are the values of STP? What is the meaning behind STP? 22. What is the lowest temperature possible? What is it called? Acids and ...
2nd Semester Final Review
... 19. If 735 L of a gas is at 3.11 atm and 34 oC, what is its temperature at 6.11 atm and 235 L? 20. If 12.2 mL of a gas is at 178oC, what is its volume at 53.0oC? 21. What are the values of STP? What is the meaning behind STP? 22. What is the lowest temperature possible? What is it called? Acids and ...
... 19. If 735 L of a gas is at 3.11 atm and 34 oC, what is its temperature at 6.11 atm and 235 L? 20. If 12.2 mL of a gas is at 178oC, what is its volume at 53.0oC? 21. What are the values of STP? What is the meaning behind STP? 22. What is the lowest temperature possible? What is it called? Acids and ...
Types of Chemical Reactions Name_________________________
... Note: Access to some of the websites may not be possible dependent upon your computer system and or the network connection. You are still required to work through each main type of reaction providing balanced chemical equations based on the word descriptions. I. How can I tell if a chemical reaction ...
... Note: Access to some of the websites may not be possible dependent upon your computer system and or the network connection. You are still required to work through each main type of reaction providing balanced chemical equations based on the word descriptions. I. How can I tell if a chemical reaction ...
Chemical reaction

A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes may occur.The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise course of action is part of the reaction mechanism. Chemical reactions are described with chemical equations, which symbolically present the starting materials, end products, and sometimes intermediate products and reaction conditions.Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration. Typically, reaction rates increase with increasing temperature because there is more thermal energy available to reach the activation energy necessary for breaking bonds between atoms.Reactions may proceed in the forward or reverse direction until they go to completion or reach equilibrium. Reactions that proceed in the forward direction to approach equilibrium are often described as spontaneous, requiring no input of free energy to go forward. Non-spontaneous reactions require input of free energy to go forward (examples include charging a battery by applying an external electrical power source, or photosynthesis driven by absorption of electromagnetic radiation in the form of sunlight).Different chemical reactions are used in combinations during chemical synthesis in order to obtain a desired product. In biochemistry, a consecutive series of chemical reactions (where the product of one reaction is the reactant of the next reaction) form metabolic pathways. These reactions are often catalyzed by protein enzymes. Enzymes increase the rates of biochemical reactions, so that metabolic syntheses and decompositions impossible under ordinary conditions can occur at the temperatures and concentrations present within a cell.The general concept of a chemical reaction has been extended to reactions between entities smaller than atoms, including nuclear reactions, radioactive decays, and reactions between elementary particles as described by quantum field theory.