The five main types of redox reactions are combination
... are those in which the oxidation states of the reactants change. This occurs because in such reactions, electrons are always transferred between species. Redox reactions take place through either a simple process, such as the burning of carbon in oxygen to yield carbon dioxide (CO2), or a more compl ...
... are those in which the oxidation states of the reactants change. This occurs because in such reactions, electrons are always transferred between species. Redox reactions take place through either a simple process, such as the burning of carbon in oxygen to yield carbon dioxide (CO2), or a more compl ...
Too Hot to Handle Lab
... reaction. The word exothermic comes from the root – “thermic”, which refers to heat, and the prefix – “exo” which means out of. Heat comes out of, or is released from, a reacting substance during an exothermic reaction. A reaction that involves burning, or a combustion reaction, is an example of an ...
... reaction. The word exothermic comes from the root – “thermic”, which refers to heat, and the prefix – “exo” which means out of. Heat comes out of, or is released from, a reacting substance during an exothermic reaction. A reaction that involves burning, or a combustion reaction, is an example of an ...
Outline
... to grams Chemical Equations A. Reactants and Products B. Balanced by atoms AND charge AND mass 1. Coefficients 2. implied “1” if nothing written a. like you to write it anyway for now 3. lowest whole number ratio C. How to balance 1. method on p137 or… 2. another way a. find biggest, ugliest molecul ...
... to grams Chemical Equations A. Reactants and Products B. Balanced by atoms AND charge AND mass 1. Coefficients 2. implied “1” if nothing written a. like you to write it anyway for now 3. lowest whole number ratio C. How to balance 1. method on p137 or… 2. another way a. find biggest, ugliest molecul ...
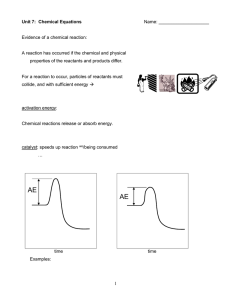
Activation energy
... • Nuclear reactions change the nucleus of an atom. • Because they affect the nucleus itself, nuclear reactions can change one element into a different element. • This means that nuclear reactions don’t balance! • Also, in nuclear reactions, some matter is converted into energy! (This is what E=mc2 m ...
... • Nuclear reactions change the nucleus of an atom. • Because they affect the nucleus itself, nuclear reactions can change one element into a different element. • This means that nuclear reactions don’t balance! • Also, in nuclear reactions, some matter is converted into energy! (This is what E=mc2 m ...
Types of Chemical Reactions
... The combustion reaction may also be an example of an earlier type such as 2Mg + O2 2MgO. The combustion reaction may be burning of a fuel. ...
... The combustion reaction may also be an example of an earlier type such as 2Mg + O2 2MgO. The combustion reaction may be burning of a fuel. ...
Chemical Equations
... Hypothetical charge use to indicate the degree of oxidation (loss of electrons) Rules in assigning oxidation states: 1) The oxidation state of a free element is zero (0). ex. O2 (g), Ag (s) 2) The oxidation state of a monatomic ion is equal to its ionic charge. (ex. Na+, Cl-3) 3) H has an oxidation ...
... Hypothetical charge use to indicate the degree of oxidation (loss of electrons) Rules in assigning oxidation states: 1) The oxidation state of a free element is zero (0). ex. O2 (g), Ag (s) 2) The oxidation state of a monatomic ion is equal to its ionic charge. (ex. Na+, Cl-3) 3) H has an oxidation ...
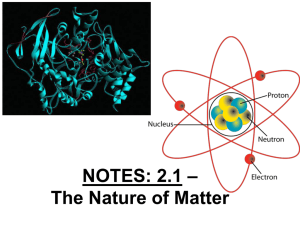
Notes
... -the number of protons in an atom of an element •all atoms of an element have the same atomic # •written as a subscript next to the element’s symbol •in a neutral atom, the number of protons is equal to the number of electrons (balanced charges). ...
... -the number of protons in an atom of an element •all atoms of an element have the same atomic # •written as a subscript next to the element’s symbol •in a neutral atom, the number of protons is equal to the number of electrons (balanced charges). ...
No Slide Title
... for 1 cup of peanut butter and all you have is ½ a cup, even though you have all the other ingredients, you can at most make ½ a batch of cookies. ...
... for 1 cup of peanut butter and all you have is ½ a cup, even though you have all the other ingredients, you can at most make ½ a batch of cookies. ...
aq - FCS Physics and Chemistry
... Cu(s) + 2AgNO3(aq) Cu(NO3)2(aq) + 2Ag(s) Not all metals will displace (react with) a metal in a compound..so how do we know if a reactions will occur? …we use our Table J in our reference tables! ...
... Cu(s) + 2AgNO3(aq) Cu(NO3)2(aq) + 2Ag(s) Not all metals will displace (react with) a metal in a compound..so how do we know if a reactions will occur? …we use our Table J in our reference tables! ...
Classifying Chemical Reactions 9-3
... We need one more oxygen in the products. Can’t change the formula, because it describes what it is (carbon monoxide in this example) ...
... We need one more oxygen in the products. Can’t change the formula, because it describes what it is (carbon monoxide in this example) ...
Chemical reaction

A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes may occur.The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise course of action is part of the reaction mechanism. Chemical reactions are described with chemical equations, which symbolically present the starting materials, end products, and sometimes intermediate products and reaction conditions.Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration. Typically, reaction rates increase with increasing temperature because there is more thermal energy available to reach the activation energy necessary for breaking bonds between atoms.Reactions may proceed in the forward or reverse direction until they go to completion or reach equilibrium. Reactions that proceed in the forward direction to approach equilibrium are often described as spontaneous, requiring no input of free energy to go forward. Non-spontaneous reactions require input of free energy to go forward (examples include charging a battery by applying an external electrical power source, or photosynthesis driven by absorption of electromagnetic radiation in the form of sunlight).Different chemical reactions are used in combinations during chemical synthesis in order to obtain a desired product. In biochemistry, a consecutive series of chemical reactions (where the product of one reaction is the reactant of the next reaction) form metabolic pathways. These reactions are often catalyzed by protein enzymes. Enzymes increase the rates of biochemical reactions, so that metabolic syntheses and decompositions impossible under ordinary conditions can occur at the temperatures and concentrations present within a cell.The general concept of a chemical reaction has been extended to reactions between entities smaller than atoms, including nuclear reactions, radioactive decays, and reactions between elementary particles as described by quantum field theory.