Multi-electron Atoms
... The capacities of these three sub-shells sum to a total of 18 electrons for the shell. The next inert gas, after neon, is argon (Z = 18). It has full K- and L-shells but only eight electrons in its M-shell corresponding to filled 3S and 3P sub-shells. The energy of the N-shell (n = 4) is close to th ...
... The capacities of these three sub-shells sum to a total of 18 electrons for the shell. The next inert gas, after neon, is argon (Z = 18). It has full K- and L-shells but only eight electrons in its M-shell corresponding to filled 3S and 3P sub-shells. The energy of the N-shell (n = 4) is close to th ...
CHAPTER 7 READING GUIDE – IONIC COMPOUNDS AND METALS
... 2. Chemical bonds can form by the attraction between the ______________ nucleus of one atom and the __________________ electrons of another atom, or by the attraction between positive ____________ and negative _____________. 3. An ______________-_________________ structure is a type of diagram used ...
... 2. Chemical bonds can form by the attraction between the ______________ nucleus of one atom and the __________________ electrons of another atom, or by the attraction between positive ____________ and negative _____________. 3. An ______________-_________________ structure is a type of diagram used ...
Prelab notes
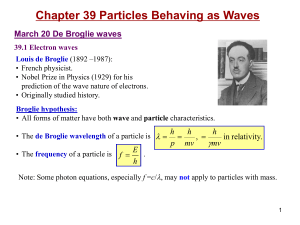
... • Davison and Germer proved this with experiments in 1927. • Why don’t we see these matter waves? Mass must be very small to observe wavelength. ...
... • Davison and Germer proved this with experiments in 1927. • Why don’t we see these matter waves? Mass must be very small to observe wavelength. ...
CH7 handout is here.
... 8. Heisenberg uncertainty principle states that we cannot know exactly the position and velocity of an electron both at the same instant. Explain what we studied under ‘position’ and under ‘velocity’. What were the assumptions when studying ‘position’? “velocity”? ...
... 8. Heisenberg uncertainty principle states that we cannot know exactly the position and velocity of an electron both at the same instant. Explain what we studied under ‘position’ and under ‘velocity’. What were the assumptions when studying ‘position’? “velocity”? ...
Electron Configuration
... • Davison and Germer proved this with experiments in 1927. • Why don’t we see these matter waves? Mass must be very small to observe wavelength. ...
... • Davison and Germer proved this with experiments in 1927. • Why don’t we see these matter waves? Mass must be very small to observe wavelength. ...
sch3u unit 1 test: matter
... 20. When baking soda is heated, sodium carbonate, water, and carbon dioxide gas are formed. This reaction can be classified as a.synthesis b.combustion c.decomposition d.single displacement ...
... 20. When baking soda is heated, sodium carbonate, water, and carbon dioxide gas are formed. This reaction can be classified as a.synthesis b.combustion c.decomposition d.single displacement ...
Topic 2 IB Chemistry Assessment Statements 2009 Revised File
... and recognize that the lines in a line spectrum are directly related to these differences. An understanding of convergence is expected. Series should be considered in the ultraviolet, visible and infrared regions of the spectrum. Calculations, knowledge of quantum numbers and historical references w ...
... and recognize that the lines in a line spectrum are directly related to these differences. An understanding of convergence is expected. Series should be considered in the ultraviolet, visible and infrared regions of the spectrum. Calculations, knowledge of quantum numbers and historical references w ...
chapter 7: atomic structure and periodicity
... Quantum numbers describe the various properties of ______________________________. 1. Principal Quantum Number – symbol – has integral values 1,2,3,….. The principal quantum number is related to the _________________ and ____________________ of the orbital. As n increases, the orbital becomes ______ ...
... Quantum numbers describe the various properties of ______________________________. 1. Principal Quantum Number – symbol – has integral values 1,2,3,….. The principal quantum number is related to the _________________ and ____________________ of the orbital. As n increases, the orbital becomes ______ ...
Chapter 4 Arrangement of Electrons in Atoms
... a photon knocks the electron off its course. • The Heisenberg uncertainty principle states that it is impossible to determine simultaneously both the position and velocity of an electron or any other particle. ...
... a photon knocks the electron off its course. • The Heisenberg uncertainty principle states that it is impossible to determine simultaneously both the position and velocity of an electron or any other particle. ...
The role of atomic radius in ion channel selectivity :
... 2. Count the total number of valence electrons. If there is a negative ion, add the absolute value of total charge to the count of valence electrons; if positive ion, subtract. 3. Count the total # of e-s needed for each atom to have a full valence shell. 4. Subtract the number in step 2 (valence ...
... 2. Count the total number of valence electrons. If there is a negative ion, add the absolute value of total charge to the count of valence electrons; if positive ion, subtract. 3. Count the total # of e-s needed for each atom to have a full valence shell. 4. Subtract the number in step 2 (valence ...
File - Chemistry 11 Enriched
... understand the location of electrons, we must now look at the atom in three dimensions rather than the planetary early model of the atom. The orbitals are not two dimensional tracks like railroads circling an atom, but are rather areas of three dimensional space where we expect to find the electron. ...
... understand the location of electrons, we must now look at the atom in three dimensions rather than the planetary early model of the atom. The orbitals are not two dimensional tracks like railroads circling an atom, but are rather areas of three dimensional space where we expect to find the electron. ...
Ionization

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons to form ions, often in conjunction with other chemical changes. Ionization can result from the loss of an electron after collisions with sub atomic particles, collisions with other atoms, molecules and ions, or through the interaction with light. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.
![L 35 Modern Physics [1]](http://s1.studyres.com/store/data/008517000_1-9aef89c0ca089782f518550164188024-300x300.png)