The Periodic Table Trends
... energy level contracts atomic radius. The greater positive charge in the nucleus the stronger the pull the nucleus has on the electrons within the existing energy level. ...
... energy level contracts atomic radius. The greater positive charge in the nucleus the stronger the pull the nucleus has on the electrons within the existing energy level. ...
Electrons in Atoms
... Each of the orbitals are characterized by a series of numbers which describe various properties of the orbital •Assigning quantum numbers are no longer covered on the AP test so this section is beyond the scope of this course. ...
... Each of the orbitals are characterized by a series of numbers which describe various properties of the orbital •Assigning quantum numbers are no longer covered on the AP test so this section is beyond the scope of this course. ...
Artificial atoms
... The confinement is accomplished with electric fields in gallium arsenide. It has a metal gate on the bottom with an insulator above it ( AlGaAs). When a positive voltage Vg is applied to the gate, electrons accumulate in the layer of GaAs above the AlGaAs. Because of the strong electric field at the ...
... The confinement is accomplished with electric fields in gallium arsenide. It has a metal gate on the bottom with an insulator above it ( AlGaAs). When a positive voltage Vg is applied to the gate, electrons accumulate in the layer of GaAs above the AlGaAs. Because of the strong electric field at the ...
ELECTRON I: Free electron model
... in this course.) Its basic assumptions are discussed in the following. (i) Independent electron approximation: There is no electron-electron interaction, although the averaged spacing is small. (ii) Free electron approximation: Ignore electron-phonon interaction. (iii) Scattering: Somehow, electrons ...
... in this course.) Its basic assumptions are discussed in the following. (i) Independent electron approximation: There is no electron-electron interaction, although the averaged spacing is small. (ii) Free electron approximation: Ignore electron-phonon interaction. (iii) Scattering: Somehow, electrons ...
Scheme for a coherently controlled pulsed electron gun F. Robicheaux
... Recent work has focused on the study of the timedependent electron emission of highly excited alkali atoms in a strong electric field using experimental1–3 and theoretical4,5 tools. The measurements on these Rydberg atoms were performed by monitoring the time-dependent flux of electrons that are nat ...
... Recent work has focused on the study of the timedependent electron emission of highly excited alkali atoms in a strong electric field using experimental1–3 and theoretical4,5 tools. The measurements on these Rydberg atoms were performed by monitoring the time-dependent flux of electrons that are nat ...
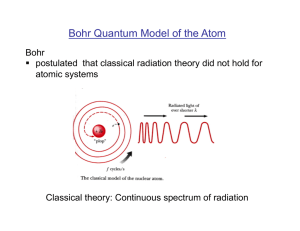
Bohr Quantum Model of the Atom
... § postulated that the electron orbital momentum is quantized Justification of Bohr’s postulates: comparison with experimental observations! ...
... § postulated that the electron orbital momentum is quantized Justification of Bohr’s postulates: comparison with experimental observations! ...
Atomic Theory
... A shielding of 0.35 is contributed by each other electron in the same group, except for a 1s electron which contributes 0.30 to the shielding of the other 1s electron For d and f electron the shielding from underlying groups is 1.00 for each electron in the underlying group. For s and p electrons th ...
... A shielding of 0.35 is contributed by each other electron in the same group, except for a 1s electron which contributes 0.30 to the shielding of the other 1s electron For d and f electron the shielding from underlying groups is 1.00 for each electron in the underlying group. For s and p electrons th ...
VIII. Other Types of Notations or Configurations
... • C. Inner electrons are called core or kernel electrons • D. Examples – Be: 1s2 2s2 – O: 1s2 2s2 2p4 – Sc: 1s2 2s2 2p6 3s2 3p6 4s2 3d1 ...
... • C. Inner electrons are called core or kernel electrons • D. Examples – Be: 1s2 2s2 – O: 1s2 2s2 2p4 – Sc: 1s2 2s2 2p6 3s2 3p6 4s2 3d1 ...
Define:
... 54. All atoms are __________ with the number of protons __________ the number of electrons. 55. What is the energy of a photon having a frequency of 3.5 x 107 Hz? 56. Which variable is directly proportional to frequency? 57. The atomic number is the total number of which particles in the nucleus? 58 ...
... 54. All atoms are __________ with the number of protons __________ the number of electrons. 55. What is the energy of a photon having a frequency of 3.5 x 107 Hz? 56. Which variable is directly proportional to frequency? 57. The atomic number is the total number of which particles in the nucleus? 58 ...
Atomic Structure
... Two types of experimental evidence which arose in the 1920s suggested an additional property of the electron. One was the closely spaced splitting of the hydrogen spectral lines, called fine structure. The other was the Stern-Gerlach experiment which showed in 1922 that a beam of silver atoms direct ...
... Two types of experimental evidence which arose in the 1920s suggested an additional property of the electron. One was the closely spaced splitting of the hydrogen spectral lines, called fine structure. The other was the Stern-Gerlach experiment which showed in 1922 that a beam of silver atoms direct ...
Hydrogen Atom Simulator – Exercises
... fraction of atoms in the Hydrogen exist at a certain energy level. This next section will explore what conditions one might expect to have if a “red” cloud of Hydrogen gas is observed in space. ...
... fraction of atoms in the Hydrogen exist at a certain energy level. This next section will explore what conditions one might expect to have if a “red” cloud of Hydrogen gas is observed in space. ...
lecture 5 radiation and matter
... Changes of energy, such as the transition of an electron from one orbit to another around the nucleus of an atom, is done in discrete quanta. Quanta are not divisible and the term quantum leap refers to the abrupt movement from one discrete energy level to another, with no smooth transition. There ...
... Changes of energy, such as the transition of an electron from one orbit to another around the nucleus of an atom, is done in discrete quanta. Quanta are not divisible and the term quantum leap refers to the abrupt movement from one discrete energy level to another, with no smooth transition. There ...
Radiation Equilibrium (in Everything Including Direct Semiconductors)
... Schrödinger equation because we already know that photons are waves described by some exp (ik·r – ωt) with ω = 2πν With that, we also know that the boundary conditions imposed by the finite crystal will only allow wave vectors that fit into the crystal and form standing waves. All we have to do then ...
... Schrödinger equation because we already know that photons are waves described by some exp (ik·r – ωt) with ω = 2πν With that, we also know that the boundary conditions imposed by the finite crystal will only allow wave vectors that fit into the crystal and form standing waves. All we have to do then ...
Chapter 8 - Fayetteville State University
... density) gives information about position momentum and energy of the electron. 5) Uncertainty principle: States that the particle-wave dualism of the electron sets an uncertainty limit for simultaneously measuring the position and momentum of the electron. 6) Emission and Absorption Spectrum: When a ...
... density) gives information about position momentum and energy of the electron. 5) Uncertainty principle: States that the particle-wave dualism of the electron sets an uncertainty limit for simultaneously measuring the position and momentum of the electron. 6) Emission and Absorption Spectrum: When a ...
HL Chemistry: Notes Atomic Theory
... a. Bohr’s was the “solar system” model of the atom. Electrons orbited the nucleus like planets orbit the sun. b. Bohr stated that electrons occupy energy levels in specific locations around the nucleus of the atom. c. The ground state is the location an unexcited electron occupies - it is the lowes ...
... a. Bohr’s was the “solar system” model of the atom. Electrons orbited the nucleus like planets orbit the sun. b. Bohr stated that electrons occupy energy levels in specific locations around the nucleus of the atom. c. The ground state is the location an unexcited electron occupies - it is the lowes ...
Ionization

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons to form ions, often in conjunction with other chemical changes. Ionization can result from the loss of an electron after collisions with sub atomic particles, collisions with other atoms, molecules and ions, or through the interaction with light. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.