UNIT 1 WORKSHEET 1. Name three methods for the separation of
... each molecule. B) there are two hydrogen atoms and one oxygen atom per water molecule. C) there is twice as much mass of oxygen as hydrogen in each molecule. D) there are two oxygen atoms and one hydrogen atom per water molecule. E) none of these ...
... each molecule. B) there are two hydrogen atoms and one oxygen atom per water molecule. C) there is twice as much mass of oxygen as hydrogen in each molecule. D) there are two oxygen atoms and one hydrogen atom per water molecule. E) none of these ...
PowerPoint
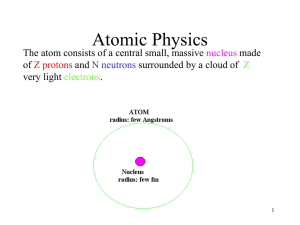
... The volume around the nucleus where the electron appears 90-95% of the time The Pauli principle No two electrons in an atom may have identical sets of four quantum numbers ...
... The volume around the nucleus where the electron appears 90-95% of the time The Pauli principle No two electrons in an atom may have identical sets of four quantum numbers ...
The Quantum Mechanical Picture of the Atom
... The volume around the nucleus where the electron appears 90-95% of the time The Pauli principle No two electrons in an atom may have identical sets of four quantum numbers ...
... The volume around the nucleus where the electron appears 90-95% of the time The Pauli principle No two electrons in an atom may have identical sets of four quantum numbers ...
Mid-Term OR Study Guide
... Be able to complete an AUFBAU CHART: Consider the element barium. Answer the following questions: (A) What is the element symbol? How many electrons are in a neutral atom of barium? (B) Draw the orbital notation for barium using up and down arrows. (C) Write the complete electron configuration for b ...
... Be able to complete an AUFBAU CHART: Consider the element barium. Answer the following questions: (A) What is the element symbol? How many electrons are in a neutral atom of barium? (B) Draw the orbital notation for barium using up and down arrows. (C) Write the complete electron configuration for b ...
Study Guide: Chapter 4 - the Arrangement of Electrons in Atoms
... 2. Understand the relationship between energy of light and its frequency; Know how to calculate the energy of light given its frequency, and the frequency given its energy (MEMORIZE FORMULA; Planck’s constant will be given on the test) – work a few practice problems 3. Know what a line emission spec ...
... 2. Understand the relationship between energy of light and its frequency; Know how to calculate the energy of light given its frequency, and the frequency given its energy (MEMORIZE FORMULA; Planck’s constant will be given on the test) – work a few practice problems 3. Know what a line emission spec ...
Chapter 6 Quiz
... ______10. When atoms share electrons, the electrical attraction of an atom for the shared electrons is called the atom's a. electron affinity. b. resonance. c. electronegativity. d. hybridization. ______11. If the atoms that share electrons have an unequal attraction for the electrons, the bond is c ...
... ______10. When atoms share electrons, the electrical attraction of an atom for the shared electrons is called the atom's a. electron affinity. b. resonance. c. electronegativity. d. hybridization. ______11. If the atoms that share electrons have an unequal attraction for the electrons, the bond is c ...
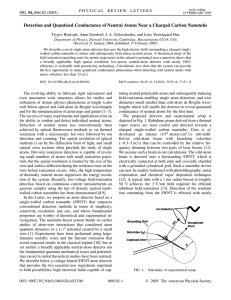
Detection and Quantized Conductance of Neutral Atoms Near a Charged... Trygve Ristroph, Anne Goodsell, J. A. Golovchenko, and Lene Vestergaard...
... The evolving ability to fabricate rigid micrometer and even nanometer scale structures allows for studies and utilization of atomic physics phenomena at length scales well below optical and cold-atom de Broglie wavelengths and for the miniaturization of atom traps and guides [1–7]. The success of ma ...
... The evolving ability to fabricate rigid micrometer and even nanometer scale structures allows for studies and utilization of atomic physics phenomena at length scales well below optical and cold-atom de Broglie wavelengths and for the miniaturization of atom traps and guides [1–7]. The success of ma ...
Chapter 5
... • DeBroglie, Einstein (and others) showed that electromagnetic radiation has properties of matter as well as waves. This is known as the waveparticle duality for light. • Wave-particle duality is perhaps one of the most confusing concepts in science, because it is so unlike anything we see in the or ...
... • DeBroglie, Einstein (and others) showed that electromagnetic radiation has properties of matter as well as waves. This is known as the waveparticle duality for light. • Wave-particle duality is perhaps one of the most confusing concepts in science, because it is so unlike anything we see in the or ...
Metal Questions
... Which statement best describes the attraction present in metallic bonding? A. the attraction between nuclei and electrons B. the attraction between positive ions and electrons C. the attraction between positive ions and negative ions D. the attraction between protons and electrons Which is the best ...
... Which statement best describes the attraction present in metallic bonding? A. the attraction between nuclei and electrons B. the attraction between positive ions and electrons C. the attraction between positive ions and negative ions D. the attraction between protons and electrons Which is the best ...
Exercises #1 - Berkeley City College
... 3. the magnetic quantum number ml describes the orientation of the orbitals in space with respect to the x-, y-, and z- coordinates. Within each sublevel, ml is allowed values from -l through 0 to +l. For example, if l = 0, ml = 0; if l = 1, ml = -1, 0, or +1; if l = 2, ml = -2, -1, 0, +1, and +2, a ...
... 3. the magnetic quantum number ml describes the orientation of the orbitals in space with respect to the x-, y-, and z- coordinates. Within each sublevel, ml is allowed values from -l through 0 to +l. For example, if l = 0, ml = 0; if l = 1, ml = -1, 0, or +1; if l = 2, ml = -2, -1, 0, +1, and +2, a ...
Chapter 5 * Electrons in Atoms
... configuration of elements? 2. Explain why the actual electron configurations for some elements differ from those assigned using the Aufbau principle. 3. Arrange the following sublevels in order of decreasing energy: 2p, 4s, 3s, 3d, and 3p. 4. Why does one electron in a potassium atom go into the fou ...
... configuration of elements? 2. Explain why the actual electron configurations for some elements differ from those assigned using the Aufbau principle. 3. Arrange the following sublevels in order of decreasing energy: 2p, 4s, 3s, 3d, and 3p. 4. Why does one electron in a potassium atom go into the fou ...
Ionization

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons to form ions, often in conjunction with other chemical changes. Ionization can result from the loss of an electron after collisions with sub atomic particles, collisions with other atoms, molecules and ions, or through the interaction with light. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.