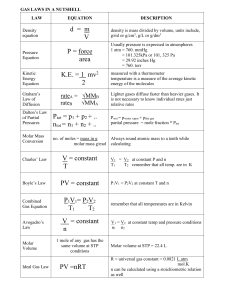
chapters 16-17 test re
... Remember to show your work as well as units. You can use one 3x5 card (front and back) on the test as notes. The only thing I will give you will be a Periodic Table. Questions #1-10 are True or False. Write True or False on the blank next to each question. 1. _______ A chemical reaction rate is defi ...
... Remember to show your work as well as units. You can use one 3x5 card (front and back) on the test as notes. The only thing I will give you will be a Periodic Table. Questions #1-10 are True or False. Write True or False on the blank next to each question. 1. _______ A chemical reaction rate is defi ...
Le Châtelier`s Principle
... Increase in the concentration of sulfur dioxide: The equilibrium shifts right to use up the extra SO2, producing more sulfur trioxide and using up some oxygen. Decreasing the partial pressure of sulfur trioxide: This is equivalent to decreasing the concentration of sulfur trioxide. The equilibrium w ...
... Increase in the concentration of sulfur dioxide: The equilibrium shifts right to use up the extra SO2, producing more sulfur trioxide and using up some oxygen. Decreasing the partial pressure of sulfur trioxide: This is equivalent to decreasing the concentration of sulfur trioxide. The equilibrium w ...
CHAPTER-7 EQUILIBRIUM Equilibrium state- When
... Ka x Kb = Kw = ionic product of water=1 x 10-14 Buffer solution :The solutions which resist change in pH on dilution or with the addition of small amounts of acid or alkali are called Buffer Solutions. common ion effect: It can be defined as a shift in equilibrium on adding a substance that pr ...
... Ka x Kb = Kw = ionic product of water=1 x 10-14 Buffer solution :The solutions which resist change in pH on dilution or with the addition of small amounts of acid or alkali are called Buffer Solutions. common ion effect: It can be defined as a shift in equilibrium on adding a substance that pr ...
practice test2
... When some of the sugar added to iced tea remains undissolved at the bottom of the glass, the solution is A) dilute B) unsaturated C) saturated D) polar ...
... When some of the sugar added to iced tea remains undissolved at the bottom of the glass, the solution is A) dilute B) unsaturated C) saturated D) polar ...
Document
... Bond formation involves the electrons (e-) in the outermost (valence) shell. A complete outer shell consists of 8 valence electrons (except H and He which have 2) Destruction of a bond corresponds to a release of energy. Generally double or triple bond energies are higher than for single bonds. ...
... Bond formation involves the electrons (e-) in the outermost (valence) shell. A complete outer shell consists of 8 valence electrons (except H and He which have 2) Destruction of a bond corresponds to a release of energy. Generally double or triple bond energies are higher than for single bonds. ...
Answer Key - La Quinta High School
... the reactant molecules combine to form the product molecules. 3. Balancing chemical equations is so important because a balanced chemical equation shows us not only the identities of the reactants and products but also the relative numbers of each involved in the process. This information is necessa ...
... the reactant molecules combine to form the product molecules. 3. Balancing chemical equations is so important because a balanced chemical equation shows us not only the identities of the reactants and products but also the relative numbers of each involved in the process. This information is necessa ...
Equilibrium
... ● Collision kinetic energy supplies potential energy that the reactants need to form products. ● Activation energy (Ea): is a threshold energy necessary for the reaction to occur. ● Collision orientation of the reactants is a reaction rate determining factor. ● Arrhenius equation: k=A , R= 8.314Jmol ...
... ● Collision kinetic energy supplies potential energy that the reactants need to form products. ● Activation energy (Ea): is a threshold energy necessary for the reaction to occur. ● Collision orientation of the reactants is a reaction rate determining factor. ● Arrhenius equation: k=A , R= 8.314Jmol ...
Lecture 6
... Stability of a phase (or mineral) is partly related to its internal energy (here “E”), which strives to be as low as possible under the external conditions. Metastability exists in a phase when its energy is higher than P-T conditions indicate it should be. (1) Activation Energy is the energy ...
... Stability of a phase (or mineral) is partly related to its internal energy (here “E”), which strives to be as low as possible under the external conditions. Metastability exists in a phase when its energy is higher than P-T conditions indicate it should be. (1) Activation Energy is the energy ...
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both reactants and products are present in concentrations which have no further tendency to change with time. Usually, this state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but equal. Thus, there are no net changes in the concentrations of the reactant(s) and product(s). Such a state is known as dynamic equilibrium.