WARM UP 9/17
... because of too many or not enough electrons (makes the atom more reactive) ANION – Too many e- , so charge is negative ...
... because of too many or not enough electrons (makes the atom more reactive) ANION – Too many e- , so charge is negative ...
Atomic Structure and the Composition of Matter
... mass and are ~1800 times more massive than the electron. Both nuclear particles are composed of quarks, smaller fundamental particles. • Protons have unit positive charge (+1), while electrons have unit negative charge (-1). Neutrons ...
... mass and are ~1800 times more massive than the electron. Both nuclear particles are composed of quarks, smaller fundamental particles. • Protons have unit positive charge (+1), while electrons have unit negative charge (-1). Neutrons ...
Chapter 3
... 1. All matter is made of indivisible and indestructible atoms. 2. All atoms of the same element are identical in their physical and chemical properties. ...
... 1. All matter is made of indivisible and indestructible atoms. 2. All atoms of the same element are identical in their physical and chemical properties. ...
ch 4 notes
... • Group # = # of valence e- (except He) – Families have similar reactivity. • Period # = # of energy levels 1A ...
... • Group # = # of valence e- (except He) – Families have similar reactivity. • Period # = # of energy levels 1A ...
Chapter 5 Notes: The Structure of Matter
... 1. Describe the reaction in words in your head 2. Write the equation using formulas and symbols if it is not already written that way 3. Check for balance with numbers under 4. Add coefficients where needed for balance ...
... 1. Describe the reaction in words in your head 2. Write the equation using formulas and symbols if it is not already written that way 3. Check for balance with numbers under 4. Add coefficients where needed for balance ...
chapter_four
... found outside the nucleus in regions called orbitals Protons are positively charged and found in the nucleus of an atom with neutrons, which have no charge There are even smaller particles but we do not study ...
... found outside the nucleus in regions called orbitals Protons are positively charged and found in the nucleus of an atom with neutrons, which have no charge There are even smaller particles but we do not study ...
Chemistry Study Guide
... Thermal energy must be added to a system for a substance to change from a solid to a liquid to a gas Or Thermal energy must be removed from a system for a substance to change from a gas to a liquid to a solid. ...
... Thermal energy must be added to a system for a substance to change from a solid to a liquid to a gas Or Thermal energy must be removed from a system for a substance to change from a gas to a liquid to a solid. ...
ChLM Final Review Name: Period: Base Knowledge 1. Classify the
... 1. Classify the following as observations or inferences a) The liquid is green because food coloring was added. b) The beaker has green liquid in it. c) The beaker can hold up to 250 mL. d) The beaker will be the best tool for this lab. 2. Measure the following, circle your estimated digit and inclu ...
... 1. Classify the following as observations or inferences a) The liquid is green because food coloring was added. b) The beaker has green liquid in it. c) The beaker can hold up to 250 mL. d) The beaker will be the best tool for this lab. 2. Measure the following, circle your estimated digit and inclu ...
CHEMISTRY 1 FINAL EXAM REVIEW
... 3.) What is the total number of atoms in one molecule of C6Hl2O6? 4.) What types of elements when combined would be most likely to form an ionic compound? 5.) What is the ionic charge on the chromium ion in the ionic compound that has the formula Cr2O3? 6.) In a polyatomic ion the -ite ending indica ...
... 3.) What is the total number of atoms in one molecule of C6Hl2O6? 4.) What types of elements when combined would be most likely to form an ionic compound? 5.) What is the ionic charge on the chromium ion in the ionic compound that has the formula Cr2O3? 6.) In a polyatomic ion the -ite ending indica ...
Physical and Chemical Properties
... • The electron cloud is the area surrounding the nucleus which contains electrons arranged in energy levels • Made mostly of empty space and almost massless electrons • Electrons are arranged in energy levels and travel in random paths around the nucleus • The number of electrons equals the number o ...
... • The electron cloud is the area surrounding the nucleus which contains electrons arranged in energy levels • Made mostly of empty space and almost massless electrons • Electrons are arranged in energy levels and travel in random paths around the nucleus • The number of electrons equals the number o ...
Document
... In figure 26, the blocks for most of the elements are _________________________. These elements are __________. The blocks for ______________ are ____________. Between the metals and the nonmetals are the _________________. These elements are represented by the _______________________________. Semim ...
... In figure 26, the blocks for most of the elements are _________________________. These elements are __________. The blocks for ______________ are ____________. Between the metals and the nonmetals are the _________________. These elements are represented by the _______________________________. Semim ...
Notes-Periodic Table (2nd Part)
... The group number (in some cases) can reveal how many electrons are in the outer most shells. Note: Electrons in the outer most shells are called valence electrons ...
... The group number (in some cases) can reveal how many electrons are in the outer most shells. Note: Electrons in the outer most shells are called valence electrons ...
Ch. 5 notes
... – Organized it by increasing atomic mass and elements with similar properties in same columns. – Able to predict future elements properties • In 1913, Moseley organized it by atomic number. ...
... – Organized it by increasing atomic mass and elements with similar properties in same columns. – Able to predict future elements properties • In 1913, Moseley organized it by atomic number. ...
Atomic Structure Video Guide
... 11. _________________ is the element that makes up graphite in a pencil. 12. If Carbon has 6 electrons then it has 6 _________________________. 13. Atomic Mass is the number of _______________________ and _____________________ in an atom. 14. Silicon (Si) is a major element that makes up ___________ ...
... 11. _________________ is the element that makes up graphite in a pencil. 12. If Carbon has 6 electrons then it has 6 _________________________. 13. Atomic Mass is the number of _______________________ and _____________________ in an atom. 14. Silicon (Si) is a major element that makes up ___________ ...
Elements and Atoms
... 3. Electrons (e-) = (negative charge) move nearly the speed of light form a cloud around the nucleus ...
... 3. Electrons (e-) = (negative charge) move nearly the speed of light form a cloud around the nucleus ...
Chemistry Vocab for Quiz 12/21 or 12/22 Atom – The smallest
... Atom – The smallest particle of an element. Atomic number - The number of protons in the nucleus of an element Atomic mass – The average mass of one atom of an element Proton – A small positively particle in the nucleus Neutron – a small particle in the nucleus with no charge Electron – A tiny negat ...
... Atom – The smallest particle of an element. Atomic number - The number of protons in the nucleus of an element Atomic mass – The average mass of one atom of an element Proton – A small positively particle in the nucleus Neutron – a small particle in the nucleus with no charge Electron – A tiny negat ...
Atomic Structure
... Electrons in the atom are to be found 90% of the time in 3D regions called orbitals THE BOHR ATOM When an electron transitions from the excited to the ground state, the atom loses energy When an electron transitions from the ground to the excited state, the atom absorbs energy Ground State Electron ...
... Electrons in the atom are to be found 90% of the time in 3D regions called orbitals THE BOHR ATOM When an electron transitions from the excited to the ground state, the atom loses energy When an electron transitions from the ground to the excited state, the atom absorbs energy Ground State Electron ...
SB Vocab list Word document
... The number of protons in the nucleus of an atom or ion. Covalent bond Sharing of a pair of electrons between non-metals Electron A negatively charged particle found in the shells of an atom. Its mass is approximately 1/2000 that of a proton or neutron. Group A vertical column in the Periodic Table. ...
... The number of protons in the nucleus of an atom or ion. Covalent bond Sharing of a pair of electrons between non-metals Electron A negatively charged particle found in the shells of an atom. Its mass is approximately 1/2000 that of a proton or neutron. Group A vertical column in the Periodic Table. ...
Honors Chemistry Chapter 6 Student Notes
... They are silvery, shiny (luster), have a low melting point, conduct electricity, and are soft (so soft, you can cut them with a knife). They are malleable (able to flattened into a sheet) and ductile (able to be drawn into a wire). Sodium and Potassium are particularly important in body chemistry. ...
... They are silvery, shiny (luster), have a low melting point, conduct electricity, and are soft (so soft, you can cut them with a knife). They are malleable (able to flattened into a sheet) and ductile (able to be drawn into a wire). Sodium and Potassium are particularly important in body chemistry. ...
STURCTURES AND PROPERTIES OF MATTER
... Carbon-14 and Carbon-13 atoms’ are not as stable as carbon-12 and easily break down. If an isotope has too many or too few neutrons compared to the number of protons, it is unstable and will undergo radioactive decay. These radioactive isotopes become different elements in an effort to become more s ...
... Carbon-14 and Carbon-13 atoms’ are not as stable as carbon-12 and easily break down. If an isotope has too many or too few neutrons compared to the number of protons, it is unstable and will undergo radioactive decay. These radioactive isotopes become different elements in an effort to become more s ...
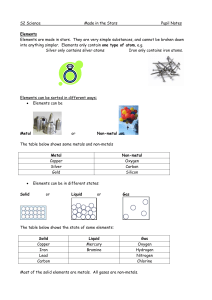
EXPERIMENT
... units that are characteristic of this element. There are different atoms for each element. In this experiment, you will be looking at some elements in the laboratory display. Some look different from each other, while others look similar. Elements can be categorized in several ways. In this experime ...
... units that are characteristic of this element. There are different atoms for each element. In this experiment, you will be looking at some elements in the laboratory display. Some look different from each other, while others look similar. Elements can be categorized in several ways. In this experime ...
Chapter 18 Notes
... -Vertical columns in the periodic table labeled as 1- 18 or I A – VIII A *Groups have similar properties, which get stronger as you move down the column. (all alkali metals react violently with water) *The closer the elements are in the column the more they have in common with each other. *Groups al ...
... -Vertical columns in the periodic table labeled as 1- 18 or I A – VIII A *Groups have similar properties, which get stronger as you move down the column. (all alkali metals react violently with water) *The closer the elements are in the column the more they have in common with each other. *Groups al ...