Chapter 1 Introduction
... reminiscent of other macrocyclic ligands. Salqu copper complexes have been investigated as oxidation catalysts[4]. Chiral versions the salen motif are derived from chiral 1,2diamines. For example, condensation of the C2-symmetric trans-1,2diaminocyclohexane with 3,5-di-tert-butylsalicylaldehdye give ...
... reminiscent of other macrocyclic ligands. Salqu copper complexes have been investigated as oxidation catalysts[4]. Chiral versions the salen motif are derived from chiral 1,2diamines. For example, condensation of the C2-symmetric trans-1,2diaminocyclohexane with 3,5-di-tert-butylsalicylaldehdye give ...
664
... Nitryl chloride may be identified by its mass spectra. The characteristic mass ions are 81, 83, 46, 35, and 37. Alternatively, nitryl chloride may be identified from its physical and chemical properties (See Reactions). The wet analytical method involves treatment with an excess solution of NaOH and ...
... Nitryl chloride may be identified by its mass spectra. The characteristic mass ions are 81, 83, 46, 35, and 37. Alternatively, nitryl chloride may be identified from its physical and chemical properties (See Reactions). The wet analytical method involves treatment with an excess solution of NaOH and ...
Answers - University of Waterloo
... *E chromium, Cr 10 Sacrificial anodes are attached to the hulls of ships to protect the iron (Fe) in the hull from corrosion. Which of the following metals could be used as a sacrificial anode for protecting the iron hull of a ship? A ...
... *E chromium, Cr 10 Sacrificial anodes are attached to the hulls of ships to protect the iron (Fe) in the hull from corrosion. Which of the following metals could be used as a sacrificial anode for protecting the iron hull of a ship? A ...
17 ADSORPTION AND CATALYSIS S MODULE - 5
... 2. Silica gel packed in small cloth bags is used for adsorbing moisture in bottles of medicine and in small electronic instruments. 3. Animal charcoal is used for decolourizing many compounds during their manufacture. 4. In chromatography, the selective adsorption of different solutes on the surface ...
... 2. Silica gel packed in small cloth bags is used for adsorbing moisture in bottles of medicine and in small electronic instruments. 3. Animal charcoal is used for decolourizing many compounds during their manufacture. 4. In chromatography, the selective adsorption of different solutes on the surface ...
CH 13
... 2. The higher the concentration of molecules, the greater the # of collisions in unit time and a faster reaction 3. As the reactants are consumed the concentration decreases, collisions decrease, reaction rate decreases 4. Reaction rate decreases with time and eventually = 0, all reactants consumed ...
... 2. The higher the concentration of molecules, the greater the # of collisions in unit time and a faster reaction 3. As the reactants are consumed the concentration decreases, collisions decrease, reaction rate decreases 4. Reaction rate decreases with time and eventually = 0, all reactants consumed ...
Carefully detach the last page. It is the Data Sheet.
... 40 Ethanoic acid, CH3COOH, is a weak acid in water. What happens when 0.01 moles of HCl are added to a 0.1 mol L−1 solution of ethanoic acid? ...
... 40 Ethanoic acid, CH3COOH, is a weak acid in water. What happens when 0.01 moles of HCl are added to a 0.1 mol L−1 solution of ethanoic acid? ...
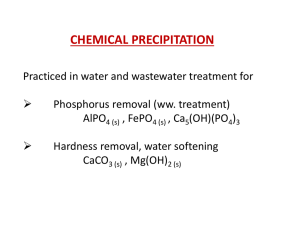
Phosphorus Removal from Wastewater by Chemical Precipitation
... Ca5(OH)(PO4)3. • Apatites are highly insoluble Ksp = 10-55.0, however a high pH must be attained to realize their formation. • Because calcium forms CaCO3 near pH 9.5 and pHs higher than 9.5 are required for significant apatite formation, the alkalinity of water is the governing factor for the requi ...
... Ca5(OH)(PO4)3. • Apatites are highly insoluble Ksp = 10-55.0, however a high pH must be attained to realize their formation. • Because calcium forms CaCO3 near pH 9.5 and pHs higher than 9.5 are required for significant apatite formation, the alkalinity of water is the governing factor for the requi ...
CHEMICAL REACTIONS Chapter 4
... Depict the kind of reactants and products and their relative amounts in a reaction. ...
... Depict the kind of reactants and products and their relative amounts in a reaction. ...
Ionic Bonding - KMChemistryMatters
... Less than an Octet • Relatively rare. • Molecules with less than an octet are typical for compounds of Groups 1A, 2A, and 3A. • Most typical example is BF3. • Formal charges indicate that the Lewis structure with an incomplete octet is more important than the ones with double bonds. ...
... Less than an Octet • Relatively rare. • Molecules with less than an octet are typical for compounds of Groups 1A, 2A, and 3A. • Most typical example is BF3. • Formal charges indicate that the Lewis structure with an incomplete octet is more important than the ones with double bonds. ...
APPLICATION OF IONIC LIQUIDS IN ORGANIC SYNTHESIS
... methodologies. These activities may provide more information on the understanding of mechanisms of organic reactions using ionic liquids as reaction media, which have been traditionally conducted in molecular solvents. It may also provide improved methodologies for product separations, particularly ...
... methodologies. These activities may provide more information on the understanding of mechanisms of organic reactions using ionic liquids as reaction media, which have been traditionally conducted in molecular solvents. It may also provide improved methodologies for product separations, particularly ...
Two-Electron Reduction of a Vanadium(V) Nitride by CO to Release
... achieved by treatment of metal complexes with azide sources and is fostered by N2 extrusion.1 The analogous transformation involving isocyanate and production of CO is less well documented. A previous study by Fickes et al. showed that the 1e reduction of a niobium(IV) isocyanate complex (OCN)Nb(N[t ...
... achieved by treatment of metal complexes with azide sources and is fostered by N2 extrusion.1 The analogous transformation involving isocyanate and production of CO is less well documented. A previous study by Fickes et al. showed that the 1e reduction of a niobium(IV) isocyanate complex (OCN)Nb(N[t ...
acid
... 2) Atoms in elemental forms have an oxidation number of 0. 3) Hydrogen has an oxidation number of +1 when bonded with nonmetals (molecular compounds) and -1 when combined with metals (ionic compounds). ...
... 2) Atoms in elemental forms have an oxidation number of 0. 3) Hydrogen has an oxidation number of +1 when bonded with nonmetals (molecular compounds) and -1 when combined with metals (ionic compounds). ...
bioinorganic 1
... In virtually all nitrogen-containing biomolecules the nitrogen is in its fully reduced form i.e. in the same oxidation state as ammonia (NH3) or ammonium cation (NH4+). The source of the nitrogen (and the principle form of the element on Earth) is dinitrogen, N2. So living systems need to convert di ...
... In virtually all nitrogen-containing biomolecules the nitrogen is in its fully reduced form i.e. in the same oxidation state as ammonia (NH3) or ammonium cation (NH4+). The source of the nitrogen (and the principle form of the element on Earth) is dinitrogen, N2. So living systems need to convert di ...
PDF File
... dissociation of P, a 5′-exon analogue shortened by one residue at its 5′-end to give CCUCdT (∆P) was used. Previous results have shown that removing the 5′-most residue of P weakens binding via base pairing with the ribozyme but does not significantly affect docking of the ribozyme-substrate duplex ...
... dissociation of P, a 5′-exon analogue shortened by one residue at its 5′-end to give CCUCdT (∆P) was used. Previous results have shown that removing the 5′-most residue of P weakens binding via base pairing with the ribozyme but does not significantly affect docking of the ribozyme-substrate duplex ...
1 chemistry of the nonmetals
... periodic tables include the element in both Group IA (with Li, Na, K, Rb, Cs, and Fr) and Group VIIA (with F, Cl, Br, I, and At). There are many reasons for including hydrogen among the elements in Group IA. It forms compounds such as HCl and HNO3 that are analogs of alkali metal compounds such as N ...
... periodic tables include the element in both Group IA (with Li, Na, K, Rb, Cs, and Fr) and Group VIIA (with F, Cl, Br, I, and At). There are many reasons for including hydrogen among the elements in Group IA. It forms compounds such as HCl and HNO3 that are analogs of alkali metal compounds such as N ...
Condition - Future Website of mrbentley2
... 1) Determine the correct Lewis structure for the molecule. If it is a diatomic (has only two atoms) it is linear. If it has 3 or more atoms continue with step 2. 2) Count the number of electron groups around the central atom. A group of electrons is a bond, a nonbonding electron pair, or occasionall ...
... 1) Determine the correct Lewis structure for the molecule. If it is a diatomic (has only two atoms) it is linear. If it has 3 or more atoms continue with step 2. 2) Count the number of electron groups around the central atom. A group of electrons is a bond, a nonbonding electron pair, or occasionall ...
chemical reactions and stoichiometry chemical reactions and
... Perkin noticed that it was coloured with a purple tinge. He washed the residue with hot alcohol and obtained a purple solution from which strikingly beautiful purple crystals precipitated. Perkin had no idea what the substance was or what reactions had created it, but he immediately saw its potentia ...
... Perkin noticed that it was coloured with a purple tinge. He washed the residue with hot alcohol and obtained a purple solution from which strikingly beautiful purple crystals precipitated. Perkin had no idea what the substance was or what reactions had created it, but he immediately saw its potentia ...
View PDF - CiteSeerX
... three object types: chromosomes and plasmids (5), genes (6) and the corresponding gene products (7). The primary link between metabolic and genomic information relates gene products that encode enzymes to the reactions these enzymes catalyze. EcoCyc also includes transport reactions and signaling p ...
... three object types: chromosomes and plasmids (5), genes (6) and the corresponding gene products (7). The primary link between metabolic and genomic information relates gene products that encode enzymes to the reactions these enzymes catalyze. EcoCyc also includes transport reactions and signaling p ...
Export To Word
... B. Electrons are key to defining chemical and some physical properties, reactivity, and molecular structures. Repeating (periodic) patterns of physical and chemical properties occur among elements that define groups of elements with similar properties. The periodic table displays the repeating patte ...
... B. Electrons are key to defining chemical and some physical properties, reactivity, and molecular structures. Repeating (periodic) patterns of physical and chemical properties occur among elements that define groups of elements with similar properties. The periodic table displays the repeating patte ...
Mock Examination (2016/2017) CHEMISTRY PAPER 1 SECTION B
... write in the margins. Answers written in the margins will not be marked. ...
... write in the margins. Answers written in the margins will not be marked. ...
Chemistry
... particle of matter. It translates to mean something that is indivisible. In the eighteenth century, chemist, John Dalton, revived the term when he suggested that each element was made up of unique atoms and the atoms of an element are all the same. At that time, there were about 35 known elements. T ...
... particle of matter. It translates to mean something that is indivisible. In the eighteenth century, chemist, John Dalton, revived the term when he suggested that each element was made up of unique atoms and the atoms of an element are all the same. At that time, there were about 35 known elements. T ...
Oxygen Reduction Reaction with the Rotating Ring Disk Electrode
... magnitude as the adsorption of hydrogen on the Pt electrode surface interferes with the ORR. The potential at the ring is fixed at 1.00 V, thus the x-axis of the graph in Figure 1 does not apply to the ring data. Although the potential of the ring is fixed, the current signal at the ring changes as ...
... magnitude as the adsorption of hydrogen on the Pt electrode surface interferes with the ORR. The potential at the ring is fixed at 1.00 V, thus the x-axis of the graph in Figure 1 does not apply to the ring data. Although the potential of the ring is fixed, the current signal at the ring changes as ...
Lewis acid catalysis
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early (titanium, zirconium) and late (iron, copper, zinc) d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen (both sp2 or sp3), nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.Many classical reactions involving carbon–carbon or carbon–heteroatom bond formation can be catalyzed by Lewis acids. Examples include the Friedel-Crafts reaction, the aldol reaction, and various pericyclic processes that proceed slowly at room temperature, such as the Diels-Alder reaction and the ene reaction. In addition to accelerating the reactions, Lewis acid catalysts are able to impose regioselectivity and stereoselectivity in many cases.Early developments in Lewis acid reagents focused on easily available compounds such as TiCl4, BF3, SnCl4, and AlCl3. The relative strengths of these (and other) Lewis acids may be estimated from NMR spectroscopy by the Childs method or the Gutmann-Beckett method. Over the years, versatile catalysts bearing ligands designed for specific applications have facilitated improvement in both reactivity and selectivity of Lewis acid-catalyzed reactions. More recently, Lewis acid catalysts with chiral ligands have become an important class of tools for asymmetric catalysis.Challenges in the development of Lewis acid catalysis include inefficient catalyst turnover (caused by catalyst affinity for the product) and the frequent requirement of two-point binding for stereoselectivity, which often necessitates the use of auxiliary groups.