Chemistry - CBSE Academic
... based, content -oriented courses are introduced. Students reach this stage after 10 years of general education and opt for Chemistry with a purpose of pursuing their career in basic sciences or professional courses like medicine, engineering, technology and study courses in applied areas of science ...
... based, content -oriented courses are introduced. Students reach this stage after 10 years of general education and opt for Chemistry with a purpose of pursuing their career in basic sciences or professional courses like medicine, engineering, technology and study courses in applied areas of science ...
data table - Tenafly Public Schools
... the same? _______________________________________________________________________ ________________________________________________________________________ 4. How many characteristic properties of two substances must be different for the two substances to be the different? ___________________________ ...
... the same? _______________________________________________________________________ ________________________________________________________________________ 4. How many characteristic properties of two substances must be different for the two substances to be the different? ___________________________ ...
Organic Reactions in Organised Media
... One of the basic requirements for every reaction is attaining proper contact between reactants. Insufficient contact between reactants will prevent the reaction from running efficiently. This is almost always the case when immiscible hydrophilic and hydrophobic reagents are involved in the process. ...
... One of the basic requirements for every reaction is attaining proper contact between reactants. Insufficient contact between reactants will prevent the reaction from running efficiently. This is almost always the case when immiscible hydrophilic and hydrophobic reagents are involved in the process. ...
Time allotted: 3hours Maximum Marks: 70
... c) Though CO is a weak Lewis base yet it forms a number of stable metal carbonyls. Explain ...
... c) Though CO is a weak Lewis base yet it forms a number of stable metal carbonyls. Explain ...
2015 chemistry
... Explain how the reaction rate is increased by this catalyst. _______________________________________________________________________________________________________ _______________________________________________________________________________________________________ _______________________________ ...
... Explain how the reaction rate is increased by this catalyst. _______________________________________________________________________________________________________ _______________________________________________________________________________________________________ _______________________________ ...
Chemistry Lab: Data Manual
... the same? _______________________________________________________________________ ________________________________________________________________________ 4. How many characteristic properties of two substances must be different for the two substances to be the different? ___________________________ ...
... the same? _______________________________________________________________________ ________________________________________________________________________ 4. How many characteristic properties of two substances must be different for the two substances to be the different? ___________________________ ...
Density functional theory and FTIR spectroscopic study of carboxyl
... such as surface science1-2, ...
... such as surface science1-2, ...
Document
... worksheet, which is meant to be used with a set of specially-colored cardboard discs, each disc representing an atom of an element indicated by the color of the disc. NOTE: As always, some of the images in this presentation have been taken from the world wide web without permission of their owners. ...
... worksheet, which is meant to be used with a set of specially-colored cardboard discs, each disc representing an atom of an element indicated by the color of the disc. NOTE: As always, some of the images in this presentation have been taken from the world wide web without permission of their owners. ...
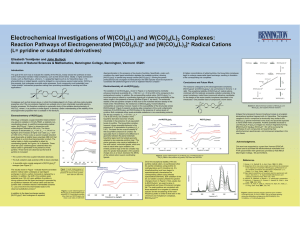
Electrochemical Investigations of W(CO) (L) and W(CO) (L) Complexes:
... response of cis-W(CO)4(py)2 and W(CO)4(bpy) in the presence of various levels of pyridine. Both complexes show quasi-reversible oxidations in non-coordinating solvents (for the bpy complex, E1/2 = 673 mV, ic/ia = 0.78 at 250 mV/s), but oxidation of the bipyridine derivative becomes virtually cis-W(C ...
... response of cis-W(CO)4(py)2 and W(CO)4(bpy) in the presence of various levels of pyridine. Both complexes show quasi-reversible oxidations in non-coordinating solvents (for the bpy complex, E1/2 = 673 mV, ic/ia = 0.78 at 250 mV/s), but oxidation of the bipyridine derivative becomes virtually cis-W(C ...
Stoichiometry File
... of chemistry involve the development of new routes to synthesize existing natural compounds. As you might imagine, making any of these syntheses commercially feasible requires detailed and quantitative understanding of the reactions involved. The economics of any chemical process obviously depend on ...
... of chemistry involve the development of new routes to synthesize existing natural compounds. As you might imagine, making any of these syntheses commercially feasible requires detailed and quantitative understanding of the reactions involved. The economics of any chemical process obviously depend on ...
CHAPTER 3 STOICHIOMETRY:
... A chemical equation must have an equal number of atoms of each element on each side of the arrow. ...
... A chemical equation must have an equal number of atoms of each element on each side of the arrow. ...
Chemistry (B) Final Exam Study Guide 3
... d. hard spheres influenced by repulsive forces from other spheres ____ 88. Which of the following statements is NOT true about the movement of particles in a gas? a. Particles travel in straight-line paths until they collide with other objects. b. Particles usually travel uninterrupted indefinitely. ...
... d. hard spheres influenced by repulsive forces from other spheres ____ 88. Which of the following statements is NOT true about the movement of particles in a gas? a. Particles travel in straight-line paths until they collide with other objects. b. Particles usually travel uninterrupted indefinitely. ...
FE Exam review for Chemistry
... and that electrons surrounded the nucleus in a diffuse cloud. The Bohr or planetary model of the atom? Bohr believed that electrons circled the nucleus only at specific, or principle, energy levels. Like planets orbiting the nucleus, sitting sun-like at the center of the atom. The quantum mechanical ...
... and that electrons surrounded the nucleus in a diffuse cloud. The Bohr or planetary model of the atom? Bohr believed that electrons circled the nucleus only at specific, or principle, energy levels. Like planets orbiting the nucleus, sitting sun-like at the center of the atom. The quantum mechanical ...
EFFECT OF LEWIS ACID IN TiCl4/MgCl2/THF/AlCl3 CATALYST
... Effect of Lewis Acid In Ticl4/Mgcl2/Thf/Alcl3 Catalyst for Ethylene Polymerization [15] J.H. Kim, "Copolymerization of ethylene and 1-butene with highly active Ti/Mg bimetallic catalysts. Effect of partial activation by AlEt2Cl,"Macromol. Rapid Commun.,vol. 16, ...
... Effect of Lewis Acid In Ticl4/Mgcl2/Thf/Alcl3 Catalyst for Ethylene Polymerization [15] J.H. Kim, "Copolymerization of ethylene and 1-butene with highly active Ti/Mg bimetallic catalysts. Effect of partial activation by AlEt2Cl,"Macromol. Rapid Commun.,vol. 16, ...
Experimental determination of hydromagnesite precipitation rates
... difference between Cl 2p electron binding energies between the fast frozen specimens and those dried under ultra-high vacuum demonstrates that the electrical double layer has been preserved and can be observed directly. The surface excess of Mg2+ and Ca2+ were measured and the organisation of cation ...
... difference between Cl 2p electron binding energies between the fast frozen specimens and those dried under ultra-high vacuum demonstrates that the electrical double layer has been preserved and can be observed directly. The surface excess of Mg2+ and Ca2+ were measured and the organisation of cation ...
5.1 questions - DrBravoChemistry
... Calculate the standard enthalpy change and the standard entropy change for this reaction. Standard enthalpy change ........................................................................... ...
... Calculate the standard enthalpy change and the standard entropy change for this reaction. Standard enthalpy change ........................................................................... ...
Problem 28. TUNNELING IN CHEMISTRY
... The natural tendency of any chemical reaction to proceed in a certain direction at constant temperature and pressure is determined by the sign of the Gibbs energy of the reaction, G. This is the universal principle. If G < 0, the reaction can proceed predominantly in the forward direction (a produ ...
... The natural tendency of any chemical reaction to proceed in a certain direction at constant temperature and pressure is determined by the sign of the Gibbs energy of the reaction, G. This is the universal principle. If G < 0, the reaction can proceed predominantly in the forward direction (a produ ...
New Materials from Metal Vapour Chemistry
... metal vapours so obtained are better than 99% atomic. In this single atom form the metal can be considered to have been artificially raised onto a "thermodynamic shelf" having its valence electrons and orbitals delicately poised for an essentially unimpeded reaction with either one of its kind, to f ...
... metal vapours so obtained are better than 99% atomic. In this single atom form the metal can be considered to have been artificially raised onto a "thermodynamic shelf" having its valence electrons and orbitals delicately poised for an essentially unimpeded reaction with either one of its kind, to f ...
semester i - Pt. Ravishankar Shukla University
... UNIT - II ELECTRONIC SPECTRA AND MAGNETIC PROPERTIES OF TRANSITION METAL COMPLEXES: Spectroscopic ground states, Correlation, Orgel and Tanabe-Sugano diagrams for transition metal complexes (d1-d9 states), Selection rules, mechanism for break down of the selection rules, intensity of absorption, ban ...
... UNIT - II ELECTRONIC SPECTRA AND MAGNETIC PROPERTIES OF TRANSITION METAL COMPLEXES: Spectroscopic ground states, Correlation, Orgel and Tanabe-Sugano diagrams for transition metal complexes (d1-d9 states), Selection rules, mechanism for break down of the selection rules, intensity of absorption, ban ...
21 More About Amines • Heterocyclic Compounds
... Amines are also exceedingly important compounds to organic chemists, far too important to leave until the end of a course in organic chemistry. We have, therefore, already studied many aspects of amines and their chemistry. For example, we have seen that the nitrogen in amines is sp 3 hybridized and ...
... Amines are also exceedingly important compounds to organic chemists, far too important to leave until the end of a course in organic chemistry. We have, therefore, already studied many aspects of amines and their chemistry. For example, we have seen that the nitrogen in amines is sp 3 hybridized and ...
File
... Write the unbalanced half-reactions for the oxidation and reduction step Balance all atoms, except H and O Balance O by adding H2O to the opposite side of the equation Balance H by adding H+ (instead of cumbersome H3O+) to the appropriate side of the equation For acidic solutions, can have H+(aq), H ...
... Write the unbalanced half-reactions for the oxidation and reduction step Balance all atoms, except H and O Balance O by adding H2O to the opposite side of the equation Balance H by adding H+ (instead of cumbersome H3O+) to the appropriate side of the equation For acidic solutions, can have H+(aq), H ...
1. Formulae, equations and amounts of substance
... Reactions where there is only one product where all atoms are used making product are ideal and have 100% atom economy. e.g. CH2=CH2 + H2 CH3CH3 ...
... Reactions where there is only one product where all atoms are used making product are ideal and have 100% atom economy. e.g. CH2=CH2 + H2 CH3CH3 ...
Lewis acid catalysis
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early (titanium, zirconium) and late (iron, copper, zinc) d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen (both sp2 or sp3), nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.Many classical reactions involving carbon–carbon or carbon–heteroatom bond formation can be catalyzed by Lewis acids. Examples include the Friedel-Crafts reaction, the aldol reaction, and various pericyclic processes that proceed slowly at room temperature, such as the Diels-Alder reaction and the ene reaction. In addition to accelerating the reactions, Lewis acid catalysts are able to impose regioselectivity and stereoselectivity in many cases.Early developments in Lewis acid reagents focused on easily available compounds such as TiCl4, BF3, SnCl4, and AlCl3. The relative strengths of these (and other) Lewis acids may be estimated from NMR spectroscopy by the Childs method or the Gutmann-Beckett method. Over the years, versatile catalysts bearing ligands designed for specific applications have facilitated improvement in both reactivity and selectivity of Lewis acid-catalyzed reactions. More recently, Lewis acid catalysts with chiral ligands have become an important class of tools for asymmetric catalysis.Challenges in the development of Lewis acid catalysis include inefficient catalyst turnover (caused by catalyst affinity for the product) and the frequent requirement of two-point binding for stereoselectivity, which often necessitates the use of auxiliary groups.