CfE Advanced Higher Chemistry
... This also provides a pattern that can often be used in identification. In both techniques some lines normally occur in the visible region (400-700 nm) but some applications use the ultraviolet region (200-400 nm). Both emission and absorption spectroscopy can be used to determine whether a certain s ...
... This also provides a pattern that can often be used in identification. In both techniques some lines normally occur in the visible region (400-700 nm) but some applications use the ultraviolet region (200-400 nm). Both emission and absorption spectroscopy can be used to determine whether a certain s ...
- sartep.com
... 55._______________Is used to explain the fact that the carbon-to-oxygen bonds in carbonate are identical 56._______________Is used to explain the fact that sodium chloride is a solid at room temperature 57._______________Is used to explain why iodine molecules are held together in the solid state 58 ...
... 55._______________Is used to explain the fact that the carbon-to-oxygen bonds in carbonate are identical 56._______________Is used to explain the fact that sodium chloride is a solid at room temperature 57._______________Is used to explain why iodine molecules are held together in the solid state 58 ...
Worksheet: Acid base problems - AP level
... 2) Determine molar amount of base required to get pH = 5.000 (for convenience, I'm going to use 1.00 L. I'll go to 250 mL at the end of this step): 1.77x + x = 0.200 x = 0.0722 mol (this is acetic acid needed in the solution) 0.200 - 0.0722 = 0.1278 mol of base required 0.1278 /4 = 0.03195 mol of ac ...
... 2) Determine molar amount of base required to get pH = 5.000 (for convenience, I'm going to use 1.00 L. I'll go to 250 mL at the end of this step): 1.77x + x = 0.200 x = 0.0722 mol (this is acetic acid needed in the solution) 0.200 - 0.0722 = 0.1278 mol of base required 0.1278 /4 = 0.03195 mol of ac ...
Reaction Kinetics - National Open University of Nigeria
... CHM 407: Reaction Kinetics concerns with the speed or rates of chemical reactions. The study of reaction rates allows for the prediction of how fast it will take a reaction mixture to reach equilibrium. It also account for how the reaction rate would be optimised by controlling certain factors such ...
... CHM 407: Reaction Kinetics concerns with the speed or rates of chemical reactions. The study of reaction rates allows for the prediction of how fast it will take a reaction mixture to reach equilibrium. It also account for how the reaction rate would be optimised by controlling certain factors such ...
Rh(acac)(CO)(PR1R2R3) - University of the Free State
... Rhodium can exist in a variety of oxidation states from +6 [RhF6] to -1 [Rh(CO)4]-. The +6, +5 and +4 states are strongly oxidising, while the Rh(III) state is the most stable. The rhodium(I) oxidation state has a d8 electron configuration and usually occurs in four-coordinate square planar structur ...
... Rhodium can exist in a variety of oxidation states from +6 [RhF6] to -1 [Rh(CO)4]-. The +6, +5 and +4 states are strongly oxidising, while the Rh(III) state is the most stable. The rhodium(I) oxidation state has a d8 electron configuration and usually occurs in four-coordinate square planar structur ...
Spontaniety Worked Examples
... (a) This process is spontaneous. Whenever two objects at different temperatures are brought into contact, heat is transferred from the hotter object to the colder one. (Section 5.1) Thus, heat is transferred from the hot metal to the cooler water. The final temperature, after the metal and water ach ...
... (a) This process is spontaneous. Whenever two objects at different temperatures are brought into contact, heat is transferred from the hotter object to the colder one. (Section 5.1) Thus, heat is transferred from the hot metal to the cooler water. The final temperature, after the metal and water ach ...
Equilibrium notes (complete)
... absorb energy from the surroundings • Some exothermic reactions don’t go to completion o Equilibrium is established as the reverse endothermic reaction occurs Randomness is a second factor that comes into play for spontaneity • Most arrangements in space are disordered • Only a few special arrangeme ...
... absorb energy from the surroundings • Some exothermic reactions don’t go to completion o Equilibrium is established as the reverse endothermic reaction occurs Randomness is a second factor that comes into play for spontaneity • Most arrangements in space are disordered • Only a few special arrangeme ...
12 - einstein classes
... is broken into small lumps and put into the ammonia convertor, where the Fe3O4 is reduced to give small crystals of iron in a refractory matrix. This is the active catalyst. The actual plant is more complicated than this one-stage reaction implies, since the N2 and H2 must be made before they can be ...
... is broken into small lumps and put into the ammonia convertor, where the Fe3O4 is reduced to give small crystals of iron in a refractory matrix. This is the active catalyst. The actual plant is more complicated than this one-stage reaction implies, since the N2 and H2 must be made before they can be ...
mole concept type 1 - teko classes bhopal
... Factor Label Method (c) POAC method } Balancing not required but common sense (d) Equivalent concept } to be discussed later ...
... Factor Label Method (c) POAC method } Balancing not required but common sense (d) Equivalent concept } to be discussed later ...
VCE Chemistry Study Design
... Chemistry is a key science in explaining the workings of our universe through an understanding of the properties and interaction of substances that make up matter. Most processes, from the formation of molecules in outer space to the complex biological interactions occurring in cells, can be describ ...
... Chemistry is a key science in explaining the workings of our universe through an understanding of the properties and interaction of substances that make up matter. Most processes, from the formation of molecules in outer space to the complex biological interactions occurring in cells, can be describ ...
Chapter - INTRODUCTION TO NANOMATERIALS
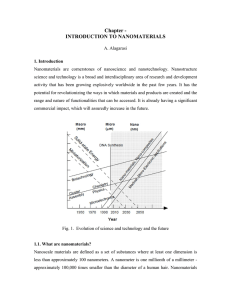
... network. This process is complicated due to fundamental changes in the structure of the gel. The drying process has itself been broken into four distinct steps: (i) the constant rate period, (ii) the critical point, (iii) the falling rate period, (iv) the second falling rate period. If isolated by t ...
... network. This process is complicated due to fundamental changes in the structure of the gel. The drying process has itself been broken into four distinct steps: (i) the constant rate period, (ii) the critical point, (iii) the falling rate period, (iv) the second falling rate period. If isolated by t ...
Praktikum in Allgemeiner Chemie für Biologen und Pharmazeuten
... solutions) or with a Bunsen burner. Large vessels (beakers, conical flasks) are heated on a support equipped with a fireproof glass plate while test tubes can be exposed directly to the flame. In order to avoid sudden eruptions of liquid during the heating of solutions in large vessels boiling aids ...
... solutions) or with a Bunsen burner. Large vessels (beakers, conical flasks) are heated on a support equipped with a fireproof glass plate while test tubes can be exposed directly to the flame. In order to avoid sudden eruptions of liquid during the heating of solutions in large vessels boiling aids ...
Chapter 16 Aqueous Ionic Equilibrium Lecture Presentation
... chemical reaction with a weak acid reactant and its conjugate base as a product. The chemical equation of a basic buffer is written with a weak base as a reactant and its conjugate acid as a product. B: + H2O H:B+ + OH− We can rewrite the Henderson–Hasselbalch equation for the chemical equation of ...
... chemical reaction with a weak acid reactant and its conjugate base as a product. The chemical equation of a basic buffer is written with a weak base as a reactant and its conjugate acid as a product. B: + H2O H:B+ + OH− We can rewrite the Henderson–Hasselbalch equation for the chemical equation of ...
Hydrocarbons and Fuels - Deans Community High School
... Each OH group in the glycerol can combine chemically with one carboxylic fatty acid molecule. The resulting molecules are fats and oils. Question: What is the name of this reaction? They are described as triglycerides. (tri esters) The hydrocarbon chain in each can be from 4 to 24 C’s long. The C’s ...
... Each OH group in the glycerol can combine chemically with one carboxylic fatty acid molecule. The resulting molecules are fats and oils. Question: What is the name of this reaction? They are described as triglycerides. (tri esters) The hydrocarbon chain in each can be from 4 to 24 C’s long. The C’s ...
Powerpoint
... Bond enthalpy is usually applied for gaseous molecules because molecules are isolated in gaseous state. In solid and liquid states, molecules are held by each other by intermolecular forces. The importance of bond enthalpy relies on the fact that it can be calculated accurately and there are some ex ...
... Bond enthalpy is usually applied for gaseous molecules because molecules are isolated in gaseous state. In solid and liquid states, molecules are held by each other by intermolecular forces. The importance of bond enthalpy relies on the fact that it can be calculated accurately and there are some ex ...
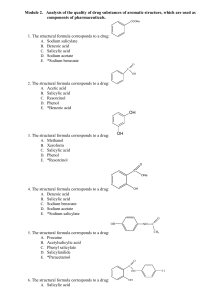
Module 2. Drug substances of aromatic structure
... E. *Bismuth tribromophenol basic with bismuth oxide 82. For synthesis of thymol it is possible to use such initial substance: A. Phenylsalicylate B. Phthalic acid C. Benzol D. Phenol E. *3-Metylphenol (m-cresol) 83. For assay of resorcinol, according to Pharmacopoeia, use method: A. Cerymetry, direc ...
... E. *Bismuth tribromophenol basic with bismuth oxide 82. For synthesis of thymol it is possible to use such initial substance: A. Phenylsalicylate B. Phthalic acid C. Benzol D. Phenol E. *3-Metylphenol (m-cresol) 83. For assay of resorcinol, according to Pharmacopoeia, use method: A. Cerymetry, direc ...
Program Review - Austin Community College
... support. Lab safety and ease of operation would be greatly improved with a larger technical staff. The Chemistry Department would like to have more technological support. We want more computers and room for the computers. In conjunction, we would like to have programs that simulate concepts taught i ...
... support. Lab safety and ease of operation would be greatly improved with a larger technical staff. The Chemistry Department would like to have more technological support. We want more computers and room for the computers. In conjunction, we would like to have programs that simulate concepts taught i ...
Exam Review Packet Table of Contents
... absent and the highest energy electrons are 3p, which has a much smaller size because the (-‐) / (+) charge ratio is less than 1 causing a contraction of the electron shell. b) Lattice energy ...
... absent and the highest energy electrons are 3p, which has a much smaller size because the (-‐) / (+) charge ratio is less than 1 causing a contraction of the electron shell. b) Lattice energy ...
Ex - Bosna Sema
... Limiting Reagent In a chemical reaction, the limiting reagent is the substance which is totally consumed when the chemical reaction is complete. The amount of product formed is limited by this reagent since the reaction cannot proceed further without it. The other reagents may be present in excess o ...
... Limiting Reagent In a chemical reaction, the limiting reagent is the substance which is totally consumed when the chemical reaction is complete. The amount of product formed is limited by this reagent since the reaction cannot proceed further without it. The other reagents may be present in excess o ...
Now! - Soojeede.com
... (b) NaOH has Na as a cation, not H (or starts with a cation other than H ) and is therefore not an acid. By writing the dissociation equation we see that NaOH is definitely not an acid. ...
... (b) NaOH has Na as a cation, not H (or starts with a cation other than H ) and is therefore not an acid. By writing the dissociation equation we see that NaOH is definitely not an acid. ...
Chapter 9 Stoichiometry
... 4. When sodium azide, NaN3 is activated in an automobile airbag, nitrogen gas and sodium are produced according the equation: 2NaN3→ 2Na + 3N2 If 0.500 mol of NaN3 react, what is the mass in grams of nitrogen would result? Given: Want: Conversion: ...
... 4. When sodium azide, NaN3 is activated in an automobile airbag, nitrogen gas and sodium are produced according the equation: 2NaN3→ 2Na + 3N2 If 0.500 mol of NaN3 react, what is the mass in grams of nitrogen would result? Given: Want: Conversion: ...
Week of Sept. 20
... Electronically tuning the metal center and using a C2 symmetric, bidentate chiral phosphine ligand led to highly enantioselective hydrogenations of enamides (very good substrates for asymmetric hydrogenations). The Monsanto Process (1974) that resulted is the 1st commercialized asymmetric synthesis ...
... Electronically tuning the metal center and using a C2 symmetric, bidentate chiral phosphine ligand led to highly enantioselective hydrogenations of enamides (very good substrates for asymmetric hydrogenations). The Monsanto Process (1974) that resulted is the 1st commercialized asymmetric synthesis ...
Lewis acid catalysis
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early (titanium, zirconium) and late (iron, copper, zinc) d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen (both sp2 or sp3), nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.Many classical reactions involving carbon–carbon or carbon–heteroatom bond formation can be catalyzed by Lewis acids. Examples include the Friedel-Crafts reaction, the aldol reaction, and various pericyclic processes that proceed slowly at room temperature, such as the Diels-Alder reaction and the ene reaction. In addition to accelerating the reactions, Lewis acid catalysts are able to impose regioselectivity and stereoselectivity in many cases.Early developments in Lewis acid reagents focused on easily available compounds such as TiCl4, BF3, SnCl4, and AlCl3. The relative strengths of these (and other) Lewis acids may be estimated from NMR spectroscopy by the Childs method or the Gutmann-Beckett method. Over the years, versatile catalysts bearing ligands designed for specific applications have facilitated improvement in both reactivity and selectivity of Lewis acid-catalyzed reactions. More recently, Lewis acid catalysts with chiral ligands have become an important class of tools for asymmetric catalysis.Challenges in the development of Lewis acid catalysis include inefficient catalyst turnover (caused by catalyst affinity for the product) and the frequent requirement of two-point binding for stereoselectivity, which often necessitates the use of auxiliary groups.