Grade XII Unit 1 - Ethiopian Ministry of Education
... (O2), nitrogen (N2), carbon dioxide (CO2), noble gases, and water vapour (H2O). Can you give more examples of mixtures? There are two broad classes of mixtures, homogeneous and heterogeneous mixtures. A homogeneous mixture is a mixture in which the composition of the mixture is the same throughout. ...
... (O2), nitrogen (N2), carbon dioxide (CO2), noble gases, and water vapour (H2O). Can you give more examples of mixtures? There are two broad classes of mixtures, homogeneous and heterogeneous mixtures. A homogeneous mixture is a mixture in which the composition of the mixture is the same throughout. ...
Chapter 3 - Educator
... The approach we have taken in arriving at balanced Equation 3.4 is largely trial and error. We balance each kind of atom in succession, adjusting coefficients as necessary. This approach works for most chemical equations. SAMPLE EXERCISE 3.1 | Interpreting and Balancing Chemical Equations The follow ...
... The approach we have taken in arriving at balanced Equation 3.4 is largely trial and error. We balance each kind of atom in succession, adjusting coefficients as necessary. This approach works for most chemical equations. SAMPLE EXERCISE 3.1 | Interpreting and Balancing Chemical Equations The follow ...
www.iitvidya.com salt analysis assignment 1. A compound on
... 39. A metallic chloride (A) does not respond chromyl chloride test. However (A) gives a white ppt. with limited amount of another metal chloride (B) and grey ppt. with excess amount of (B). (A) when treated with KI gives a scarlet ppt. which dissolves in excess of Ki forming an important reagent (C) ...
... 39. A metallic chloride (A) does not respond chromyl chloride test. However (A) gives a white ppt. with limited amount of another metal chloride (B) and grey ppt. with excess amount of (B). (A) when treated with KI gives a scarlet ppt. which dissolves in excess of Ki forming an important reagent (C) ...
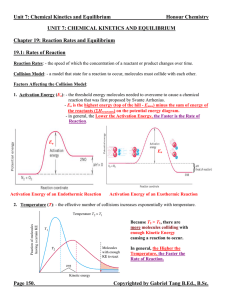
Unit 7 Reaction Rates and Equilibrium Notes
... Le Châtelier’s Principle: - a qualitative method to predict the shift on an equilibrium system if it is disturbed by means of changing concentration, pressure and temperature. - the equilibrium will shift in the direction that minimizes the change imposed on the system. 1. Effects of a Change in Con ...
... Le Châtelier’s Principle: - a qualitative method to predict the shift on an equilibrium system if it is disturbed by means of changing concentration, pressure and temperature. - the equilibrium will shift in the direction that minimizes the change imposed on the system. 1. Effects of a Change in Con ...
Side Chain Chemistry Mediates Backbone Fragmentation in
... A crown ether based, photolabile radical precursor which forms noncovalent complexes with peptides has been prepared. The peptide/precursor complexes can be electrosprayed, isolated in an ion trap, and then subjected to laser photolysis and collision induced dissociation to generate hydrogen deficie ...
... A crown ether based, photolabile radical precursor which forms noncovalent complexes with peptides has been prepared. The peptide/precursor complexes can be electrosprayed, isolated in an ion trap, and then subjected to laser photolysis and collision induced dissociation to generate hydrogen deficie ...
van Geel workbook 2012
... Chapter 4: Empirical and Molecular Formulas Find the empirical formulas for the following compounds. 1. C2H2 ____________________ 2. C6H12O6 ____________________ 3. C3H4 ____________________ 4. C4H6 ____________________ 5. C12H22O11 ____________________ 6. The empirical formula of a compound is NO2, ...
... Chapter 4: Empirical and Molecular Formulas Find the empirical formulas for the following compounds. 1. C2H2 ____________________ 2. C6H12O6 ____________________ 3. C3H4 ____________________ 4. C4H6 ____________________ 5. C12H22O11 ____________________ 6. The empirical formula of a compound is NO2, ...
Limiting Reactants and Percentage Yield
... Once one of the reactants is used up, no more product can be formed. The substance that is completely used up first in a reaction is called the limiting reactant. The limiting reactant is the reactant that limits the amount of the other reactant that can combine and the amount of product that can fo ...
... Once one of the reactants is used up, no more product can be formed. The substance that is completely used up first in a reaction is called the limiting reactant. The limiting reactant is the reactant that limits the amount of the other reactant that can combine and the amount of product that can fo ...
Application of Novel Phosphine Ligands in Palladium
... considered as the world’s leading catalyst designer providing the most selective, active and complex catalysts. Biocatalysis is often defined as a ‘blend’ of homogeneous and heterogeneous catalysis; especially enzymes are macromolecules, and therefore it is sometimes difficult to decide whether they ...
... considered as the world’s leading catalyst designer providing the most selective, active and complex catalysts. Biocatalysis is often defined as a ‘blend’ of homogeneous and heterogeneous catalysis; especially enzymes are macromolecules, and therefore it is sometimes difficult to decide whether they ...
10. Solution Guide to Supplementary Exercises
... Four different flasks, A, B, C and D, at the same temperature, contain a mixture of PCl5(g), PCl3(g) and Cl2(g). The concentration, in mol dm–3, of these components in each of the flasks is shown below. In three of the four flasks, the mixture of gases is at equilibrium. In which one is the mixture ...
... Four different flasks, A, B, C and D, at the same temperature, contain a mixture of PCl5(g), PCl3(g) and Cl2(g). The concentration, in mol dm–3, of these components in each of the flasks is shown below. In three of the four flasks, the mixture of gases is at equilibrium. In which one is the mixture ...
Kinetic Assay of Human Pepsin with Albumin
... comparisons with results from other investigative centers. In general, research groups have tended to establish their own reference ranges for the given experiment, with the result that the reported values often do not have universal applicability. Moreover, no pure, stable human pepsin is yet avail ...
... comparisons with results from other investigative centers. In general, research groups have tended to establish their own reference ranges for the given experiment, with the result that the reported values often do not have universal applicability. Moreover, no pure, stable human pepsin is yet avail ...
A photochemical model for the Venus atmosphere at 47–112 km
... The model is intended to respond to the recent findings in the Venus atmosphere from the Venus Express and ground-based submillimeter and infrared observations. It extends down to 47 km for comparison with the kinetic model for the lower atmosphere (Krasnopolsky, V.A. [2007]. Icarus 191, 25–37) and t ...
... The model is intended to respond to the recent findings in the Venus atmosphere from the Venus Express and ground-based submillimeter and infrared observations. It extends down to 47 km for comparison with the kinetic model for the lower atmosphere (Krasnopolsky, V.A. [2007]. Icarus 191, 25–37) and t ...
Go FIGure
... Liquids that mix in all proportions, such as acetone and water, are miscible, whereas those that do not dissolve in one another are immiscible. Gasoline, which is a mixture of hydrocarbons, is immiscible with water. Hydrocarbons are nonpolar substances because of several factors: The C ¬ C bonds are ...
... Liquids that mix in all proportions, such as acetone and water, are miscible, whereas those that do not dissolve in one another are immiscible. Gasoline, which is a mixture of hydrocarbons, is immiscible with water. Hydrocarbons are nonpolar substances because of several factors: The C ¬ C bonds are ...
GEOCHEMICAL AND BIOGEOCHEMICAL
... purposes. Within a few months of its completion the software was in use at dozens of universities and companies around the world. We find that the programs allow us to try fresh approaches to teaching aqueous geochemistry. Once a student can reliably balance reactions by hand, the task quickly become ...
... purposes. Within a few months of its completion the software was in use at dozens of universities and companies around the world. We find that the programs allow us to try fresh approaches to teaching aqueous geochemistry. Once a student can reliably balance reactions by hand, the task quickly become ...
Equilibrium Part 2
... at equilibrium, constitutes a stress. Adding more reactant upsets the established equilibrium. Let’s look at this imaginary equilibrium system where the size of the symbol represents the concentration of that reactant or product. ...
... at equilibrium, constitutes a stress. Adding more reactant upsets the established equilibrium. Let’s look at this imaginary equilibrium system where the size of the symbol represents the concentration of that reactant or product. ...
AP Chemistry - Siva Kodali
... 2,000 students per year. She is the author of ACT For Dummies, Pre-Calculus For Dummies, AP Biology For Dummies, Chemistry Workbook For Dummies, GRE For Dummies, Pre-Calculus Workbook for Dummies, and other books on self-esteem, writing, and motivational topics. Michelle has overseen dozens of progr ...
... 2,000 students per year. She is the author of ACT For Dummies, Pre-Calculus For Dummies, AP Biology For Dummies, Chemistry Workbook For Dummies, GRE For Dummies, Pre-Calculus Workbook for Dummies, and other books on self-esteem, writing, and motivational topics. Michelle has overseen dozens of progr ...
purdue university - IUPUI ScholarWorks
... Figure 5.1 FT-IR spectra of pure CTAB (a) and mesoporous chromium phosphate prepared with an atomic ratio of P/Cr = 2.0 before (b) and after (c) the surfactant removal. ........................................................ 50 Figure 5.2 FT-IR spectra of mesoporous chromium phosphate prepared with ...
... Figure 5.1 FT-IR spectra of pure CTAB (a) and mesoporous chromium phosphate prepared with an atomic ratio of P/Cr = 2.0 before (b) and after (c) the surfactant removal. ........................................................ 50 Figure 5.2 FT-IR spectra of mesoporous chromium phosphate prepared with ...
THE SYNTHESIS AND CHARACTERIZATION OF PHOSPHONIUM INDENYLIDE COMPLEXES OF RUTHENIUM(II)
... found teaching that lab rewarding and I think I learned as much as my students did. There have been too many people whom I have crossed paths with to name that have made Queen’s the center of many fond memories. The regulars at Jay’s Bar and Grill, the Uehehe’s, Andrew Fraser, Jason Hanthorn, and Be ...
... found teaching that lab rewarding and I think I learned as much as my students did. There have been too many people whom I have crossed paths with to name that have made Queen’s the center of many fond memories. The regulars at Jay’s Bar and Grill, the Uehehe’s, Andrew Fraser, Jason Hanthorn, and Be ...
- Boreskov Institute of Catalysis
... The Boreskov Institute of Catalysis, or BIC, is well known to experts in both academic and industrial catalysis not only in Russia and the fSU countries, but also in many western and oriental countries. It is the world oldest institution which was organized to provide any kind of R&D in the area of ...
... The Boreskov Institute of Catalysis, or BIC, is well known to experts in both academic and industrial catalysis not only in Russia and the fSU countries, but also in many western and oriental countries. It is the world oldest institution which was organized to provide any kind of R&D in the area of ...
Massachusetts Tests for Educator Licensure (MTEL )
... Correct Response: B. The use of lightweight plastic materials for automobile parts reduces the overall weight of a vehicle. Less overall weight leads to an increase in fuel efficiency which reduces the amount of carbon dioxide emitted into the atmosphere. A is incorrect because the metal parts that ...
... Correct Response: B. The use of lightweight plastic materials for automobile parts reduces the overall weight of a vehicle. Less overall weight leads to an increase in fuel efficiency which reduces the amount of carbon dioxide emitted into the atmosphere. A is incorrect because the metal parts that ...
Answer Key - mrkelleher
... Name _______________________________ Date __________________ Class __________________ therefore +3 each. nitrogen(III) oxide 7. a. Carbon is +4 and each oxygen is 2. b. Carbon is 4 and each hydrogen is +1. c. Each carbon is 0, each hydrogen is +1, and each oxygen is 2. d. Each carbon is 8/3 and ...
... Name _______________________________ Date __________________ Class __________________ therefore +3 each. nitrogen(III) oxide 7. a. Carbon is +4 and each oxygen is 2. b. Carbon is 4 and each hydrogen is +1. c. Each carbon is 0, each hydrogen is +1, and each oxygen is 2. d. Each carbon is 8/3 and ...
View/Open
... The magnitudes of changes observed under the two conditions are different. ∆E) is the heat change accompanying a chemical reaction at The change in internal energy (∆ constant volume because no external work is performed. However at constant pressure not only does the change in internal energy take ...
... The magnitudes of changes observed under the two conditions are different. ∆E) is the heat change accompanying a chemical reaction at The change in internal energy (∆ constant volume because no external work is performed. However at constant pressure not only does the change in internal energy take ...
Lewis acid catalysis
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early (titanium, zirconium) and late (iron, copper, zinc) d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen (both sp2 or sp3), nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.Many classical reactions involving carbon–carbon or carbon–heteroatom bond formation can be catalyzed by Lewis acids. Examples include the Friedel-Crafts reaction, the aldol reaction, and various pericyclic processes that proceed slowly at room temperature, such as the Diels-Alder reaction and the ene reaction. In addition to accelerating the reactions, Lewis acid catalysts are able to impose regioselectivity and stereoselectivity in many cases.Early developments in Lewis acid reagents focused on easily available compounds such as TiCl4, BF3, SnCl4, and AlCl3. The relative strengths of these (and other) Lewis acids may be estimated from NMR spectroscopy by the Childs method or the Gutmann-Beckett method. Over the years, versatile catalysts bearing ligands designed for specific applications have facilitated improvement in both reactivity and selectivity of Lewis acid-catalyzed reactions. More recently, Lewis acid catalysts with chiral ligands have become an important class of tools for asymmetric catalysis.Challenges in the development of Lewis acid catalysis include inefficient catalyst turnover (caused by catalyst affinity for the product) and the frequent requirement of two-point binding for stereoselectivity, which often necessitates the use of auxiliary groups.