Chemistry - Department of Education and Skills
... authors have found beneficial over the years and gives details of student experiments and teacher demonstrations. Worked examples are included which the teacher may find useful in the class room or for homework. It is not intended that this book be used as a textbook or be read from cover to cover. ...
... authors have found beneficial over the years and gives details of student experiments and teacher demonstrations. Worked examples are included which the teacher may find useful in the class room or for homework. It is not intended that this book be used as a textbook or be read from cover to cover. ...
Review Study Guide for the Final
... What is Periodic Law? When elements are arrange in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties. ...
... What is Periodic Law? When elements are arrange in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties. ...
Module 2
... Helmholtz’ free energy. Termodynamical equilibrium conditions. The criteria for the spontaneous processes direction. The basic principles of thermodynamics applying to living organisms. ATP as an energy source for biochemical reactions. Macroergic compounds. 3.2. Overview Definition of the first law ...
... Helmholtz’ free energy. Termodynamical equilibrium conditions. The criteria for the spontaneous processes direction. The basic principles of thermodynamics applying to living organisms. ATP as an energy source for biochemical reactions. Macroergic compounds. 3.2. Overview Definition of the first law ...
Chapter 12
... You are given moles of the reactant propane, and moles of the product carbon dioxide must be found. The balanced chemical equation must be written. Conversion from moles of C3H8 to moles of CO2 is required. The correct mole ratio has moles of unknown substance in the numerator and moles of known sub ...
... You are given moles of the reactant propane, and moles of the product carbon dioxide must be found. The balanced chemical equation must be written. Conversion from moles of C3H8 to moles of CO2 is required. The correct mole ratio has moles of unknown substance in the numerator and moles of known sub ...
AS/A level
... Further evidence for this model comes from successive ionisation energies. Explain how these provide evidence for aspects of the model described. Sketch the expected pattern of successive ionisation energies for an atom of aluminium and use it to illustrate your answer. ...
... Further evidence for this model comes from successive ionisation energies. Explain how these provide evidence for aspects of the model described. Sketch the expected pattern of successive ionisation energies for an atom of aluminium and use it to illustrate your answer. ...
CLUE - virtual laboratories
... chemistry textbook? The answer is, of course not—not if that book is just a variation on those currently available. Chemistry, and particularly introductory general chemistry, is simply not changing that much and people learn pretty much the same way they always did, at least if we restrict ourselve ...
... chemistry textbook? The answer is, of course not—not if that book is just a variation on those currently available. Chemistry, and particularly introductory general chemistry, is simply not changing that much and people learn pretty much the same way they always did, at least if we restrict ourselve ...
Modern inorganic chemistry
... We now know of the existence of over one hundred elements. A century ago, more than sixty of these were already known, and naturally attempts were made to relate the properties of all these elements in some way. One obvious method was to classify them as metals and non-metals; but this clearly did n ...
... We now know of the existence of over one hundred elements. A century ago, more than sixty of these were already known, and naturally attempts were made to relate the properties of all these elements in some way. One obvious method was to classify them as metals and non-metals; but this clearly did n ...
New liquid absorbents for the removal of CO2 from gas
... experimental results have shown that among the studied carriers, arginine and ornithine are the natural amino acids with greater affinity towards CO2 and that some of the here synthesized amino acids show outstanding absorption capacities, superior to MEA or any other amino acid tested. The develope ...
... experimental results have shown that among the studied carriers, arginine and ornithine are the natural amino acids with greater affinity towards CO2 and that some of the here synthesized amino acids show outstanding absorption capacities, superior to MEA or any other amino acid tested. The develope ...
Conformational studies of aliphatic secondary ozonides
... factors. Foremost, the five membered ring can adopt various puckering forms. The conformations of such a ring can be classified into an envelope (E) form in which one atom lies out of the plane of the other atoms, and twist (T) form in which two consecutive atoms lie on opposite faces of the plane of ...
... factors. Foremost, the five membered ring can adopt various puckering forms. The conformations of such a ring can be classified into an envelope (E) form in which one atom lies out of the plane of the other atoms, and twist (T) form in which two consecutive atoms lie on opposite faces of the plane of ...
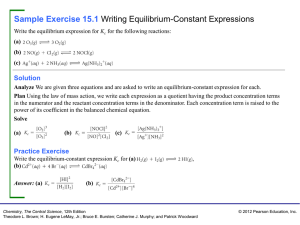
Sample Exercise 15.1 Writing Equilibrium
... Plan For equilibrium to be achieved, it must be possible for both the forward process and the reverse process to occur. For the forward process to occur, there must be some calcium carbonate present. For the reverse process to occur, there must be both calcium oxide and carbon dioxide. In both cases ...
... Plan For equilibrium to be achieved, it must be possible for both the forward process and the reverse process to occur. For the forward process to occur, there must be some calcium carbonate present. For the reverse process to occur, there must be both calcium oxide and carbon dioxide. In both cases ...
Chapter 4 Classifying Reactions: Chemicals in Balance
... Pb(NO3)2(aq) + Mg(s) → Mg(NO3)2(aq) + Pb(s) ...
... Pb(NO3)2(aq) + Mg(s) → Mg(NO3)2(aq) + Pb(s) ...
Unit 5 Organic Chemistry
... fuels. They are also the primary sources of hydrocarbons—compounds containing carbon atoms bonded to hydrogen atoms. Hydrocarbons are the starting points in the synthesis of thousands of products, including specific fuels, plastics, and synthetic fibres. Some hydrocarbons are obtained directly by ph ...
... fuels. They are also the primary sources of hydrocarbons—compounds containing carbon atoms bonded to hydrogen atoms. Hydrocarbons are the starting points in the synthesis of thousands of products, including specific fuels, plastics, and synthetic fibres. Some hydrocarbons are obtained directly by ph ...
Syllabus and Regulations for 2-year, 4
... 2. The course will consist of 12 papers of 75/80/85/90/100-marks (Group-A: Theoretical-50 Marks and Group-B: Practical- 25/30/35/40/50-Marks) each. The examiners shall forward assessment in respect of every candidate to the Principal / Controller of Examination / Coordinator P. G. Courses (as the ca ...
... 2. The course will consist of 12 papers of 75/80/85/90/100-marks (Group-A: Theoretical-50 Marks and Group-B: Practical- 25/30/35/40/50-Marks) each. The examiners shall forward assessment in respect of every candidate to the Principal / Controller of Examination / Coordinator P. G. Courses (as the ca ...
Document
... Suppose Si (having 4 valence electrons is doped with Ga (which has 3 valence electrons), 3 valence electrons are involved in bond formation with neighboring Si atom. A vacancy is left which can be filled by the transfer of a valence electron from a neighboring Si atom. The movement of electron into ...
... Suppose Si (having 4 valence electrons is doped with Ga (which has 3 valence electrons), 3 valence electrons are involved in bond formation with neighboring Si atom. A vacancy is left which can be filled by the transfer of a valence electron from a neighboring Si atom. The movement of electron into ...
BSC with Chemistry CBCS Syllabus 2016-17
... constant and ionic product of water. Ionization of weak acids and bases, pH scale, common ion effect. Salt hydrolysis-calculation of hydrolysis constant, degree of hydrolysis and pH for different salts. Buffer solutions. Solubility and solubility product of sparingly soluble salts – applications of ...
... constant and ionic product of water. Ionization of weak acids and bases, pH scale, common ion effect. Salt hydrolysis-calculation of hydrolysis constant, degree of hydrolysis and pH for different salts. Buffer solutions. Solubility and solubility product of sparingly soluble salts – applications of ...
chemistry - The Aga Khan University
... Trends in Density 13.2.2 Trends in Reactivity with Water 13.2.3 Reactions with Oxygen 13.2.3.1 Reactions with Air or Oxygen and the formation of Normal Oxides, Peroxides, Super Oxides and their Stability 13.2.3.2 Reactions of Oxides with Water and Dilute Acids 13.2.4 Reactions with Chlorine 13.2.5 E ...
... Trends in Density 13.2.2 Trends in Reactivity with Water 13.2.3 Reactions with Oxygen 13.2.3.1 Reactions with Air or Oxygen and the formation of Normal Oxides, Peroxides, Super Oxides and their Stability 13.2.3.2 Reactions of Oxides with Water and Dilute Acids 13.2.4 Reactions with Chlorine 13.2.5 E ...
Continued on Next page
... Step 2 Determine the number of moles of HNO3 (or KOH) reacted. Multiply by the given ∆H to find the enthalpy corresponding to the number of moles reacted. Since Qrxn = −Qsoln , state Q as a positive value. Remember to convert kJ to J. Step 3 Rearrange the equation Q = m · c · ∆T to calculate the ∆T ...
... Step 2 Determine the number of moles of HNO3 (or KOH) reacted. Multiply by the given ∆H to find the enthalpy corresponding to the number of moles reacted. Since Qrxn = −Qsoln , state Q as a positive value. Remember to convert kJ to J. Step 3 Rearrange the equation Q = m · c · ∆T to calculate the ∆T ...
Test
... There will be no net gain in either product or reactant. e) The equilibrium constant will decrease until it equals the reaction quotient. ...
... There will be no net gain in either product or reactant. e) The equilibrium constant will decrease until it equals the reaction quotient. ...
File
... 36. Hydrogen reacts with some elements to form binary compounds called __________. (Halides, Hydrides, Oxides, all of these) 37. The hydrides formed by the transfer of electrons from electropositive metals to hydrogen are called __________. (Ionic hydrides, covalent hydrides, Complex hydrides, Inter ...
... 36. Hydrogen reacts with some elements to form binary compounds called __________. (Halides, Hydrides, Oxides, all of these) 37. The hydrides formed by the transfer of electrons from electropositive metals to hydrogen are called __________. (Ionic hydrides, covalent hydrides, Complex hydrides, Inter ...
chemical equilibrium type 1
... Most of the chemical reaction do not go to completion in a closed system and attain a state of equilibrium. Equilibrium is said to have reached in a physical or chemical system when rate of forward and reverse processes are equal. At equilibrium macroscopic properties of the system like concentratio ...
... Most of the chemical reaction do not go to completion in a closed system and attain a state of equilibrium. Equilibrium is said to have reached in a physical or chemical system when rate of forward and reverse processes are equal. At equilibrium macroscopic properties of the system like concentratio ...
APPROACHES TO CARBOHYDRATE-BASED CHEMICAL LIBRARIES: THE
... of intermediates, and thus combinatorial chemistry places a great emphasis on performing reactions that proceed in high yields. ...
... of intermediates, and thus combinatorial chemistry places a great emphasis on performing reactions that proceed in high yields. ...
Lewis acid catalysis
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early (titanium, zirconium) and late (iron, copper, zinc) d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen (both sp2 or sp3), nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.Many classical reactions involving carbon–carbon or carbon–heteroatom bond formation can be catalyzed by Lewis acids. Examples include the Friedel-Crafts reaction, the aldol reaction, and various pericyclic processes that proceed slowly at room temperature, such as the Diels-Alder reaction and the ene reaction. In addition to accelerating the reactions, Lewis acid catalysts are able to impose regioselectivity and stereoselectivity in many cases.Early developments in Lewis acid reagents focused on easily available compounds such as TiCl4, BF3, SnCl4, and AlCl3. The relative strengths of these (and other) Lewis acids may be estimated from NMR spectroscopy by the Childs method or the Gutmann-Beckett method. Over the years, versatile catalysts bearing ligands designed for specific applications have facilitated improvement in both reactivity and selectivity of Lewis acid-catalyzed reactions. More recently, Lewis acid catalysts with chiral ligands have become an important class of tools for asymmetric catalysis.Challenges in the development of Lewis acid catalysis include inefficient catalyst turnover (caused by catalyst affinity for the product) and the frequent requirement of two-point binding for stereoselectivity, which often necessitates the use of auxiliary groups.