class XI CHEMISTRY - Kendriya Vidyalaya No.1 Harni Road
... Chemistry: Chemistry is the branch of science that deals with the composition,structure and properties of matter. Chemistry is called the science of atoms and molecule Branches of Chemistry Organic Chemistry -This branch deals with study of carbon compounds especially hydrocarbons and their deriva ...
... Chemistry: Chemistry is the branch of science that deals with the composition,structure and properties of matter. Chemistry is called the science of atoms and molecule Branches of Chemistry Organic Chemistry -This branch deals with study of carbon compounds especially hydrocarbons and their deriva ...
- Kendriya Vidyalaya No. 2 Raipur
... Chemistry: Chemistry is the branch of science that deals with the composition,structure and properties of matter. Chemistry is called the science of atoms and molecule Branches of Chemistry Organic Chemistry -This branch deals with study of carbon compounds especially hydrocarbons and their deriva ...
... Chemistry: Chemistry is the branch of science that deals with the composition,structure and properties of matter. Chemistry is called the science of atoms and molecule Branches of Chemistry Organic Chemistry -This branch deals with study of carbon compounds especially hydrocarbons and their deriva ...
class XI CHEMISTRY - Kendriya Vidyalaya No.1 Ichhanath Surat
... Chemistry: Chemistry is the branch of science that deals with the composition,structure and properties of matter. Chemistry is called the science of atoms and molecule Branches of Chemistry Organic Chemistry -This branch deals with study of carbon compounds especially hydrocarbons and their deriva ...
... Chemistry: Chemistry is the branch of science that deals with the composition,structure and properties of matter. Chemistry is called the science of atoms and molecule Branches of Chemistry Organic Chemistry -This branch deals with study of carbon compounds especially hydrocarbons and their deriva ...
Quiz contsts questions chemistry
... Q 20. When a metal carbonate weighing 0.84g was heated strongly, 0.40 g of metal oxide was left as the residue. The specific heat of the metals 0.25. The atomic weight of the metal is : (a) ...
... Q 20. When a metal carbonate weighing 0.84g was heated strongly, 0.40 g of metal oxide was left as the residue. The specific heat of the metals 0.25. The atomic weight of the metal is : (a) ...
Chapter 5 Geochemical Weathering
... Weathering reactions and the consumption of acidity The primary aqueous reactions in groundwater systems that produce acidity involve atmospheric oxygen and some reduced compound such as organic carbon. Bacterially-mediated respiration is the most important. Respiration takes place mainly in soil wa ...
... Weathering reactions and the consumption of acidity The primary aqueous reactions in groundwater systems that produce acidity involve atmospheric oxygen and some reduced compound such as organic carbon. Bacterially-mediated respiration is the most important. Respiration takes place mainly in soil wa ...
Η - Knockhardy
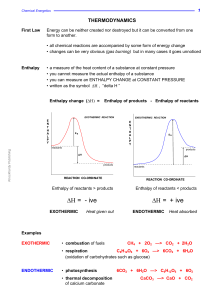
... Imagine that, during a reaction, all the bonds of reacting species are broken and the individual atoms join up again but in the form of products. The overall energy change will depend on the difference between the energy required to break the bonds and that released as bonds are made. energy release ...
... Imagine that, during a reaction, all the bonds of reacting species are broken and the individual atoms join up again but in the form of products. The overall energy change will depend on the difference between the energy required to break the bonds and that released as bonds are made. energy release ...
STOICHIOMETRY
... obtained assuming that all the starting materials react completely and no product is lost. The balanced equation always gives the theoretical yield. In many industrial processes and laboratory reactions the actual yield obtained is significantly less than the theoretical yield. The relationship betw ...
... obtained assuming that all the starting materials react completely and no product is lost. The balanced equation always gives the theoretical yield. In many industrial processes and laboratory reactions the actual yield obtained is significantly less than the theoretical yield. The relationship betw ...
B.Sc Chemistry - Calicut University
... behaviour of individual atoms and molecules. The laws of quantum mechanics decide the properties of the micro-world. There are two approaches for introducing quantum mechanics. One is to follow the historical development of the quantum theory and the other is to begin from the basic principles of th ...
... behaviour of individual atoms and molecules. The laws of quantum mechanics decide the properties of the micro-world. There are two approaches for introducing quantum mechanics. One is to follow the historical development of the quantum theory and the other is to begin from the basic principles of th ...
Chapter 09 An Overview of Chemical Reactions Notes
... Precipitation Reaction: - a reaction where a precipitate (new solid) is formed as a product. Neutralization Reaction: - a reaction between an acid and a base where water is formed as a product. To Predict Products and Balance Chemical Equations: 1. Write the correct chemical formulas for all product ...
... Precipitation Reaction: - a reaction where a precipitate (new solid) is formed as a product. Neutralization Reaction: - a reaction between an acid and a base where water is formed as a product. To Predict Products and Balance Chemical Equations: 1. Write the correct chemical formulas for all product ...
Nitrogen and Oxygen Family
... or R3P=CH2 (R = alkyl group). Phosphorus and arsenic can form d–p bond also with transition metals when their compounds like P(C2H5)3 and As(C6H5)3 act as ligands. Hydrides : All the elements of Group 15 form hydrides of the type EH3 where E=N, P, As, Sb or Bi. Some of the properties of these hydr ...
... or R3P=CH2 (R = alkyl group). Phosphorus and arsenic can form d–p bond also with transition metals when their compounds like P(C2H5)3 and As(C6H5)3 act as ligands. Hydrides : All the elements of Group 15 form hydrides of the type EH3 where E=N, P, As, Sb or Bi. Some of the properties of these hydr ...
Guide Kjeldahl
... In the past 200 years the techniques supporting chemical analysis have made tremendous progress from the invention of the Bunsen-burner and its use for flame tests to the atomic force microscope sent to Mars for the exploration of martian soil. At the time when Johan Kjeldahl published his method fo ...
... In the past 200 years the techniques supporting chemical analysis have made tremendous progress from the invention of the Bunsen-burner and its use for flame tests to the atomic force microscope sent to Mars for the exploration of martian soil. At the time when Johan Kjeldahl published his method fo ...
Document
... group is MORE positive than it was in the C-O-H group By increasing the number of highly electronegative O, more electrons were drawn away from that C, making it more positive. ...
... group is MORE positive than it was in the C-O-H group By increasing the number of highly electronegative O, more electrons were drawn away from that C, making it more positive. ...
OCR A Level Chemistry A H432 Specification
... interesting to teach and administer within all centres (large and small). Our new A Level in Chemistry A builds on our existing popular course. We’ve based the redevelopment of our A level sciences on an understanding of what works well in centres large and small and have updated areas of content an ...
... interesting to teach and administer within all centres (large and small). Our new A Level in Chemistry A builds on our existing popular course. We’ve based the redevelopment of our A level sciences on an understanding of what works well in centres large and small and have updated areas of content an ...
1. Blood cholesterol levels are generally expressed as milligrams of
... but the denities of the gases are different because mass of 1 mol N2 = 28.014 g per mol and molar mass of He = 4.0026 g per mol so the density of the N2 gas = 28.014 g / 22.4 L = 1.25 g per / L and density of the Helium = 4.0026 g / 22.4 L = 0.179 g / L Therefore as the when the balloon is filled wi ...
... but the denities of the gases are different because mass of 1 mol N2 = 28.014 g per mol and molar mass of He = 4.0026 g per mol so the density of the N2 gas = 28.014 g / 22.4 L = 1.25 g per / L and density of the Helium = 4.0026 g / 22.4 L = 0.179 g / L Therefore as the when the balloon is filled wi ...
Study Material - Class- XI- Chemistry
... Chemistry: Chemistry is the branch of science that deals with the composition,structure and properties of matter. Chemistry is called the science of atoms and molecules. Branches of Chemistry Organic Chemistry -This branch deals with study of carbon compounds especially hydrocarbons and their deri ...
... Chemistry: Chemistry is the branch of science that deals with the composition,structure and properties of matter. Chemistry is called the science of atoms and molecules. Branches of Chemistry Organic Chemistry -This branch deals with study of carbon compounds especially hydrocarbons and their deri ...
2.6 M - Thierry Karsenti
... If you get 6 items or more correct you can consider that you are doing fine, but if you get less than 4 items correct then you have to work very hard to pass the course. ...
... If you get 6 items or more correct you can consider that you are doing fine, but if you get less than 4 items correct then you have to work very hard to pass the course. ...
Question Bank - Edudel.nic.in
... Translate the following statements into chemical equations and balance them :(i) Sodium chloride reacts with silver nitrate to give sodium nitrate and a precipitate of silver chloride. (ii) Hydrogen gas combines with nitrogen to form ammonia. (iii) Zinc carbonate decomposes to give zinc oxide and ca ...
... Translate the following statements into chemical equations and balance them :(i) Sodium chloride reacts with silver nitrate to give sodium nitrate and a precipitate of silver chloride. (ii) Hydrogen gas combines with nitrogen to form ammonia. (iii) Zinc carbonate decomposes to give zinc oxide and ca ...
Document
... reaction is started with [H2 ]0 = 0.76 M, [N2]0 = 0.60 M and [NH3]0= 0.48 M. Which of the following is correct as the reaction comes to equilibrium? A) The concentration of N2will increase B) The concentration of H2will decrease C) The concentration of NH3will decrease D) The concentration of both N ...
... reaction is started with [H2 ]0 = 0.76 M, [N2]0 = 0.60 M and [NH3]0= 0.48 M. Which of the following is correct as the reaction comes to equilibrium? A) The concentration of N2will increase B) The concentration of H2will decrease C) The concentration of NH3will decrease D) The concentration of both N ...
Chapter 14
... Because there are standard ways of find the change in entropy for a pure substance as we change the temperature of the substance at constant pressure, the third law of thermodynamics allows us to assign values for entropy for pure substances at any temperature. Standard molar entropy (S) – The valu ...
... Because there are standard ways of find the change in entropy for a pure substance as we change the temperature of the substance at constant pressure, the third law of thermodynamics allows us to assign values for entropy for pure substances at any temperature. Standard molar entropy (S) – The valu ...
Chapter 4 "Reactions in Aqueous Solution"
... solutes2, are dispersed uniformly throughout the substance in the greater amount, the solvent3. An aqueous solution4 is a solution in which the solvent is water, whereas in a nonaqueous solution, the solvent is a substance other than water. Familiar examples of nonaqueous solvents are ethyl acetate, ...
... solutes2, are dispersed uniformly throughout the substance in the greater amount, the solvent3. An aqueous solution4 is a solution in which the solvent is water, whereas in a nonaqueous solution, the solvent is a substance other than water. Familiar examples of nonaqueous solvents are ethyl acetate, ...
Lewis acid catalysis
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early (titanium, zirconium) and late (iron, copper, zinc) d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen (both sp2 or sp3), nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.Many classical reactions involving carbon–carbon or carbon–heteroatom bond formation can be catalyzed by Lewis acids. Examples include the Friedel-Crafts reaction, the aldol reaction, and various pericyclic processes that proceed slowly at room temperature, such as the Diels-Alder reaction and the ene reaction. In addition to accelerating the reactions, Lewis acid catalysts are able to impose regioselectivity and stereoselectivity in many cases.Early developments in Lewis acid reagents focused on easily available compounds such as TiCl4, BF3, SnCl4, and AlCl3. The relative strengths of these (and other) Lewis acids may be estimated from NMR spectroscopy by the Childs method or the Gutmann-Beckett method. Over the years, versatile catalysts bearing ligands designed for specific applications have facilitated improvement in both reactivity and selectivity of Lewis acid-catalyzed reactions. More recently, Lewis acid catalysts with chiral ligands have become an important class of tools for asymmetric catalysis.Challenges in the development of Lewis acid catalysis include inefficient catalyst turnover (caused by catalyst affinity for the product) and the frequent requirement of two-point binding for stereoselectivity, which often necessitates the use of auxiliary groups.