UNIVERSITY OF DELHI FACULTY OF SCIENCE SYLLABUS OF COURSES TO BE OFFERED
... diatomic and simple polyatomic molecules N2, O2, C2, B2, F2, CO, NO, and their ions; HCl (idea of s-p mixing and orbital interaction to be given). Formal charge, Valence shell electron pair repulsion theory (VSEPR), shapes of the following simple molecules and ions containing lone pairs and bond pai ...
... diatomic and simple polyatomic molecules N2, O2, C2, B2, F2, CO, NO, and their ions; HCl (idea of s-p mixing and orbital interaction to be given). Formal charge, Valence shell electron pair repulsion theory (VSEPR), shapes of the following simple molecules and ions containing lone pairs and bond pai ...
Answers
... the empirical formula, which shows only the simplest whole number ratio of one atom to another. It conveys the least information about a molecule. ...
... the empirical formula, which shows only the simplest whole number ratio of one atom to another. It conveys the least information about a molecule. ...
Chemistry
... of oxymercuration-demercuration, hydroboration- oxidation, ozonolysis, reduction (catalytic and chemical), syn and anti-hydroxylation(oxidation). 1,2-and 1,4-addition reactions in conjugated dienes and Diels-Alder reaction; Allylic and benzylic bromination and mechanism, e.g. propene, 1-butene, tolu ...
... of oxymercuration-demercuration, hydroboration- oxidation, ozonolysis, reduction (catalytic and chemical), syn and anti-hydroxylation(oxidation). 1,2-and 1,4-addition reactions in conjugated dienes and Diels-Alder reaction; Allylic and benzylic bromination and mechanism, e.g. propene, 1-butene, tolu ...
Specification and sample assessment material - Edexcel
... understand that the noble gases (Group 0) are a family of inert gases and explain their lack of reactivity in terms of their electronic configurations. ...
... understand that the noble gases (Group 0) are a family of inert gases and explain their lack of reactivity in terms of their electronic configurations. ...
Soln Chem 2008Nov(9746)
... Boiling point increases from C2H5Cl to C2H5I due to stronger intermolecular van der Waals' forces as the number of electrons increases from C2H5Cl to C2H5I. (ans) (ii) bond polarity: C–Cl > C–Br > C–I Bond polarity decreases from C–Cl to C–I due to decrease in electronegativity from Cl to I. (ans) ...
... Boiling point increases from C2H5Cl to C2H5I due to stronger intermolecular van der Waals' forces as the number of electrons increases from C2H5Cl to C2H5I. (ans) (ii) bond polarity: C–Cl > C–Br > C–I Bond polarity decreases from C–Cl to C–I due to decrease in electronegativity from Cl to I. (ans) ...
Chemistry.of Organic Compounds
... reactions take place, that is, with the mechanism of the reactions. The introduction of this material into basic organic textbooks, however, has been very slow. The essential facts regarding the mechanism of the addition of hydrogen cyanide to aldehydes, for example, were reported by Lapworth in 190 ...
... reactions take place, that is, with the mechanism of the reactions. The introduction of this material into basic organic textbooks, however, has been very slow. The essential facts regarding the mechanism of the addition of hydrogen cyanide to aldehydes, for example, were reported by Lapworth in 190 ...
CHAPTER 16 ACID-BASE EQUILIBRIA AND SOLUBILITY
... Dissolving 0.20 M sodium acetate initially produces 0.20 M CH3COO and 0.20 M Na . The sodium ions are not involved in any further equilibrium (why?), but the acetate ions must be added to the equilibrium in part (a). CH3COOH(aq) Initial (M): Change (M): Equilibrium (M): ...
... Dissolving 0.20 M sodium acetate initially produces 0.20 M CH3COO and 0.20 M Na . The sodium ions are not involved in any further equilibrium (why?), but the acetate ions must be added to the equilibrium in part (a). CH3COOH(aq) Initial (M): Change (M): Equilibrium (M): ...
The chemistry of beer aging – a critical review Food Chemistry
... detectable levels, as research on beer carbonyls is complicated due the extremely low levels at which many of these compounds occur. However, it is questionable whether the results are representative of real storage conditions. In general, it remains important that steps in the analytical procedure ...
... detectable levels, as research on beer carbonyls is complicated due the extremely low levels at which many of these compounds occur. However, it is questionable whether the results are representative of real storage conditions. In general, it remains important that steps in the analytical procedure ...
Review of N and Metal co-Doped TiO for Water Purification under
... pairs resulting in enhancement of photocatalytic activity for both oxidation and reduction. Apart from doping TiO2 with a single metal or nonmetal, there are several efforts that combine the two approaches, which have been reported that could result in inducing synergistic effects and higher visible ...
... pairs resulting in enhancement of photocatalytic activity for both oxidation and reduction. Apart from doping TiO2 with a single metal or nonmetal, there are several efforts that combine the two approaches, which have been reported that could result in inducing synergistic effects and higher visible ...
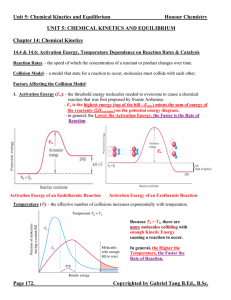
Unit 5: Chemical Kinetics and Equilibrium
... 1. When K >> 1, the equilibrium system favours the products. There are more products than reactants at the state of equilibrium. ([C]eq and [D]eq or PC, eq and PD, eq >> [A]eq and [B]eq or PA, eq and PB, eq) 2. When K << 1, the equilibrium system favours the reactants. There are less products than r ...
... 1. When K >> 1, the equilibrium system favours the products. There are more products than reactants at the state of equilibrium. ([C]eq and [D]eq or PC, eq and PD, eq >> [A]eq and [B]eq or PA, eq and PB, eq) 2. When K << 1, the equilibrium system favours the reactants. There are less products than r ...
Sam P. de Visser,* Jan-Uwe Rohde,* Yong
... Single crystals of the triflate salt of this highly oxidized complex were obtained at −40 ◦ C, and its structure was established by Xray crystallography (Fig. 3a), which revealed an Fe O distance of 1.646(3) Å [40]. This very short distance is consistent with strong and bonding between the Fe ce ...
... Single crystals of the triflate salt of this highly oxidized complex were obtained at −40 ◦ C, and its structure was established by Xray crystallography (Fig. 3a), which revealed an Fe O distance of 1.646(3) Å [40]. This very short distance is consistent with strong and bonding between the Fe ce ...
The Impact of Ligand Design on the Coordination Chemistry and
... Figure 4.2. (a) Structure of fac-ReBr(CO)3[H(LMe)], 1Me (b) Structure of the cation in {fac-Re(CO)3[H(LMe)]}(PF6), 2Me (c) Structure of fac-e(CO)3(LMe),3Me....................87 Figure 4.3.(a) Structure of H(LMe) (b) Structure of fac-ReBr(CO)3[H(LiPr)], 1iPr (c) Structure of cation in {fac-Re(CO)3[H ...
... Figure 4.2. (a) Structure of fac-ReBr(CO)3[H(LMe)], 1Me (b) Structure of the cation in {fac-Re(CO)3[H(LMe)]}(PF6), 2Me (c) Structure of fac-e(CO)3(LMe),3Me....................87 Figure 4.3.(a) Structure of H(LMe) (b) Structure of fac-ReBr(CO)3[H(LiPr)], 1iPr (c) Structure of cation in {fac-Re(CO)3[H ...
1 Solutions 4a (Chapter 4 problems) Chem151 [Kua]
... (c) The second two parts of this problem involve stoichiometric calculations. The problem gives information about the amounts of both starting materials, so this is a limiting reactant situation. We must calculate the number of moles of each species, construct a table of amounts, and use the result ...
... (c) The second two parts of this problem involve stoichiometric calculations. The problem gives information about the amounts of both starting materials, so this is a limiting reactant situation. We must calculate the number of moles of each species, construct a table of amounts, and use the result ...
Ch 18 Power Point
... • Catalysts have no effect on relative equilibrium amounts. • They only affect the rates at which equilibrium is reached. • Catalysts increase the rates of forward and reverse reactions in a system by equal factors. Therefore, they do not affect K. ...
... • Catalysts have no effect on relative equilibrium amounts. • They only affect the rates at which equilibrium is reached. • Catalysts increase the rates of forward and reverse reactions in a system by equal factors. Therefore, they do not affect K. ...
Study Guide for Chapter 22 - Hydrocarbon Compounds
... • Because carbon has four valence electrons, carbon atoms always form four covalent bonds. • The carbon atoms in an alkane can be arranged in a straight chain or in a chain that has branches. • Molecules of hydrocarbons, such as alkanes, are nonpolar molecules. ...
... • Because carbon has four valence electrons, carbon atoms always form four covalent bonds. • The carbon atoms in an alkane can be arranged in a straight chain or in a chain that has branches. • Molecules of hydrocarbons, such as alkanes, are nonpolar molecules. ...
Chapter 15
... There are occasions when the use of an equilibrium arrow is not appropriate. For example, when hydrogen and oxygen react to form water vapor (Figure 15.X), product formation is very strongly favored and no noticeable amounts of reactants are formed by the reverse reaction. The chemical equation repr ...
... There are occasions when the use of an equilibrium arrow is not appropriate. For example, when hydrogen and oxygen react to form water vapor (Figure 15.X), product formation is very strongly favored and no noticeable amounts of reactants are formed by the reverse reaction. The chemical equation repr ...
Chapter 15: Chemical Equilibrium
... There are occasions when the use of an equilibrium arrow is not appropriate. For example, when hydrogen and oxygen react to form water vapor (Figure 15.X), product formation is very strongly favored and no noticeable amounts of reactants are formed by the reverse reaction. The chemical equation repr ...
... There are occasions when the use of an equilibrium arrow is not appropriate. For example, when hydrogen and oxygen react to form water vapor (Figure 15.X), product formation is very strongly favored and no noticeable amounts of reactants are formed by the reverse reaction. The chemical equation repr ...
Solutions Manual
... Cellulose is made from repeating units of β-glucose with inversion of every second unit. This produces long, straight chains of cellulose which are linked to each other by hydrogen bonding. In plants, cellulose acts as a structural material. Starch (both amylase and amylopectin) is made from long-ch ...
... Cellulose is made from repeating units of β-glucose with inversion of every second unit. This produces long, straight chains of cellulose which are linked to each other by hydrogen bonding. In plants, cellulose acts as a structural material. Starch (both amylase and amylopectin) is made from long-ch ...
Stoichiometry
... 6.0221x1023 = NA is Avogadro's number. A mole is used to indicate a number of atoms just as a dozen is used to indicate a number of eggs. Converting from moles to atoms is done the same as converting dozens to items. 1.5 doz = (1.5 doz)(12 items.doz-1) = 18 items and 1.5 mol = (1.5 mol)( 6.0x1023 at ...
... 6.0221x1023 = NA is Avogadro's number. A mole is used to indicate a number of atoms just as a dozen is used to indicate a number of eggs. Converting from moles to atoms is done the same as converting dozens to items. 1.5 doz = (1.5 doz)(12 items.doz-1) = 18 items and 1.5 mol = (1.5 mol)( 6.0x1023 at ...
Polyhedral Oligomeric Silsesquioxane
... as long as the amount of catalyst is kept between 0.01 and 0.1 mol %. However, iodide ion is not a strong stabilizer for Pd(0) NPs so as to produce the low reactive precipitate of Pd black. Thus, a stable ligand is still necessary for the design of a recyclable catalyst. Polyhedral oligosilsesquioxa ...
... as long as the amount of catalyst is kept between 0.01 and 0.1 mol %. However, iodide ion is not a strong stabilizer for Pd(0) NPs so as to produce the low reactive precipitate of Pd black. Thus, a stable ligand is still necessary for the design of a recyclable catalyst. Polyhedral oligosilsesquioxa ...
Lewis acid catalysis
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early (titanium, zirconium) and late (iron, copper, zinc) d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen (both sp2 or sp3), nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.Many classical reactions involving carbon–carbon or carbon–heteroatom bond formation can be catalyzed by Lewis acids. Examples include the Friedel-Crafts reaction, the aldol reaction, and various pericyclic processes that proceed slowly at room temperature, such as the Diels-Alder reaction and the ene reaction. In addition to accelerating the reactions, Lewis acid catalysts are able to impose regioselectivity and stereoselectivity in many cases.Early developments in Lewis acid reagents focused on easily available compounds such as TiCl4, BF3, SnCl4, and AlCl3. The relative strengths of these (and other) Lewis acids may be estimated from NMR spectroscopy by the Childs method or the Gutmann-Beckett method. Over the years, versatile catalysts bearing ligands designed for specific applications have facilitated improvement in both reactivity and selectivity of Lewis acid-catalyzed reactions. More recently, Lewis acid catalysts with chiral ligands have become an important class of tools for asymmetric catalysis.Challenges in the development of Lewis acid catalysis include inefficient catalyst turnover (caused by catalyst affinity for the product) and the frequent requirement of two-point binding for stereoselectivity, which often necessitates the use of auxiliary groups.













![1 Solutions 4a (Chapter 4 problems) Chem151 [Kua]](http://s1.studyres.com/store/data/002731518_1-574ec10e88e667508364281b6325aeef-300x300.png)