Chapter 8 Review Sheet
... 41. Identify at least five endergonic reactions in the cell that must be powered using the exergonic reaction of ATP hydrolysis. 42. Why do we breathe in air for molecular oxygen (O2)? 43. Describe the three major fates of the food that you eat. Which are exergonic and which are endergonic? Which ar ...
... 41. Identify at least five endergonic reactions in the cell that must be powered using the exergonic reaction of ATP hydrolysis. 42. Why do we breathe in air for molecular oxygen (O2)? 43. Describe the three major fates of the food that you eat. Which are exergonic and which are endergonic? Which ar ...
James W. Whittaker - Oxygen reactions of the copper oxidases
... Each of the three redox forms of dioxygen (molecular oxygen, superoxide and hydrogen peroxide) has specific interactions with biological systems [2,3]. Dioxygen itself can bind reversibly with oxygen carriers, such as haemoglobin (the haemoprotein of mammalian blood), haemerythrin (a non-haem iron p ...
... Each of the three redox forms of dioxygen (molecular oxygen, superoxide and hydrogen peroxide) has specific interactions with biological systems [2,3]. Dioxygen itself can bind reversibly with oxygen carriers, such as haemoglobin (the haemoprotein of mammalian blood), haemerythrin (a non-haem iron p ...
Macromolecules of Life – Lecture 1
... Chlorophyll and carotenoid pigments are located in the membranes of the thylakoids. ...
... Chlorophyll and carotenoid pigments are located in the membranes of the thylakoids. ...
CHAPTER 4 Energy transfers and transformations
... Compartment IV, the PHOTOAUTOTROPHIC compartment: Inputs: Minerals and nitrates (from Compartment III) and carbon dioxide (from Compartments I and V). Active occupants: This compartment (IV) is composed of two sections (Compartments IVA and IVB). • Spiral cyanobacteria, Arthrospira platensis, commo ...
... Compartment IV, the PHOTOAUTOTROPHIC compartment: Inputs: Minerals and nitrates (from Compartment III) and carbon dioxide (from Compartments I and V). Active occupants: This compartment (IV) is composed of two sections (Compartments IVA and IVB). • Spiral cyanobacteria, Arthrospira platensis, commo ...
Chapter 20 Notes
... An oxidation involving FAD • Mechanism involves hydride removal by FAD and a deprotonation • This enzyme is actually part of the electron transport pathway in the inner mitochondrial membrane • The electrons transferred from succinate to FAD (to form FADH2) are passed directly to ubiquinone (UQ) in ...
... An oxidation involving FAD • Mechanism involves hydride removal by FAD and a deprotonation • This enzyme is actually part of the electron transport pathway in the inner mitochondrial membrane • The electrons transferred from succinate to FAD (to form FADH2) are passed directly to ubiquinone (UQ) in ...
Photosynthesis self
... The acceptor passes it to NADP+, which becomes reduced to NADPH. According to the following equation, NADP+ has the capacity to carry two electrons. NADP+ + 2e- + H+ NADPH Thylakoids ...
... The acceptor passes it to NADP+, which becomes reduced to NADPH. According to the following equation, NADP+ has the capacity to carry two electrons. NADP+ + 2e- + H+ NADPH Thylakoids ...
Section 6.1 Summary – pages 141-151
... across space is called a concentration gradient. • Ions and molecules diffuse from an area of higher concentration to an area of lower concentration, moving with the gradient. ...
... across space is called a concentration gradient. • Ions and molecules diffuse from an area of higher concentration to an area of lower concentration, moving with the gradient. ...
Scholarly Interest Report
... The discipline of bioenergetics attempts to characterize the biochemical processes whereby the chemical free energy that originates with our diet is made available to living organisms. In eucaryotic systems the relevant processes are catalyzed by enzyme complexes present in the inner membrane of the ...
... The discipline of bioenergetics attempts to characterize the biochemical processes whereby the chemical free energy that originates with our diet is made available to living organisms. In eucaryotic systems the relevant processes are catalyzed by enzyme complexes present in the inner membrane of the ...
outlines
... Active Transport - works against the concentration gradient and requires energy (ATP hydrolysis) 1) Primary (ex. Na/K pump—3Na+ pumped out and 2K+ pumped into cell fueled by ATP hydrolysis) 2) Secondary – use one solutes gradient to accomplish the transport of another (Na+/glucose cotransport) 3) Io ...
... Active Transport - works against the concentration gradient and requires energy (ATP hydrolysis) 1) Primary (ex. Na/K pump—3Na+ pumped out and 2K+ pumped into cell fueled by ATP hydrolysis) 2) Secondary – use one solutes gradient to accomplish the transport of another (Na+/glucose cotransport) 3) Io ...
Slide 1
... ETC As electrons pass down chain, H+ ions are pumped into inter-mitochondrial space making a charge gradient. Gradient provides energy for ATP synthase to add the P group. At the end of the chain, an enzyme combines the electrons with H+ and oxygen to form water, a by-product of electron ...
... ETC As electrons pass down chain, H+ ions are pumped into inter-mitochondrial space making a charge gradient. Gradient provides energy for ATP synthase to add the P group. At the end of the chain, an enzyme combines the electrons with H+ and oxygen to form water, a by-product of electron ...
Answers
... How is Anaerobic Respiration different from Fermentation? Indicate all that apply. a. Fermentation has no ETC b. Oxygen is not required c. The final electron acceptor in fermentation is an organic molecule d. Fermentation does not produce any ATP ANSWER BACK TO GAME ...
... How is Anaerobic Respiration different from Fermentation? Indicate all that apply. a. Fermentation has no ETC b. Oxygen is not required c. The final electron acceptor in fermentation is an organic molecule d. Fermentation does not produce any ATP ANSWER BACK TO GAME ...
Lab
... from the food they eat. Plants obtain this energy from sunlight and convert it into sugars in the process called photosynthesis. Plants capture sunlight using chlorophyll molecules found in the chloroplasts of their cells. Chlorophyll gives plants their green color. The highest concentration of chlo ...
... from the food they eat. Plants obtain this energy from sunlight and convert it into sugars in the process called photosynthesis. Plants capture sunlight using chlorophyll molecules found in the chloroplasts of their cells. Chlorophyll gives plants their green color. The highest concentration of chlo ...
Allied Biochemistry II - E
... 4. The components of respiratory chain are arranged in the order of (a) increasing redox potential (b) decreasing redox potential (c) independent of redox potential (d) none of the above 5. The common metabolite of carbohydrate, protein and lipid metabolism (a) acetyl CoA (b) pyruvate (c) fumarate ...
... 4. The components of respiratory chain are arranged in the order of (a) increasing redox potential (b) decreasing redox potential (c) independent of redox potential (d) none of the above 5. The common metabolite of carbohydrate, protein and lipid metabolism (a) acetyl CoA (b) pyruvate (c) fumarate ...
Fat-Soluble
... damage to membranes by free radicals. Free radicals are molecules with an unpaired (extra/missing) electron, which can be passed on to other molecules, leading to a chain reaction that damages cells. Free radicals are a normal product of respiration. • Also needed for optimal nerve transmission alon ...
... damage to membranes by free radicals. Free radicals are molecules with an unpaired (extra/missing) electron, which can be passed on to other molecules, leading to a chain reaction that damages cells. Free radicals are a normal product of respiration. • Also needed for optimal nerve transmission alon ...
Chapter 18
... phosphopantetheine are carriers of acyl groups which are attached in thiolester linkage to the terminal SH. The thiol esters ha ve high negative free energies of hydrolysis, and they also help to labilize the hydrogens on the alpha carbon. 5’Deoxyadenosylcobalamin has a carbon-cobalt bond, and it is ...
... phosphopantetheine are carriers of acyl groups which are attached in thiolester linkage to the terminal SH. The thiol esters ha ve high negative free energies of hydrolysis, and they also help to labilize the hydrogens on the alpha carbon. 5’Deoxyadenosylcobalamin has a carbon-cobalt bond, and it is ...
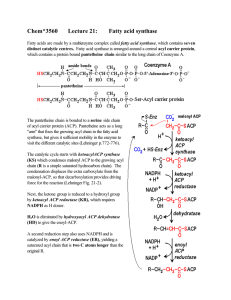
Chem*3560 Lecture 21: Fatty acid synthase
... The enzyme makes oxaloacetate as a bound intermediate, and then decarboxylates it to release pyruvate as a product. The decarboxylation provides the driving force needed to produce excess NADPH. Enz:[oxaloacetate] → pyruvate + CO2 Many organisms also contain an NADP + dependent isozyme of isocitrate ...
... The enzyme makes oxaloacetate as a bound intermediate, and then decarboxylates it to release pyruvate as a product. The decarboxylation provides the driving force needed to produce excess NADPH. Enz:[oxaloacetate] → pyruvate + CO2 Many organisms also contain an NADP + dependent isozyme of isocitrate ...
Bioenergetics and Metabolism
... Glycolysis is the sole source of ATP under anaerobic conditions which can occur in animal muscle tissue during intense exercise. Fermentation also relies on glycolysis which is a process that is used to make alcoholic beverages when yeast cells are provided glucose without oxygen. ...
... Glycolysis is the sole source of ATP under anaerobic conditions which can occur in animal muscle tissue during intense exercise. Fermentation also relies on glycolysis which is a process that is used to make alcoholic beverages when yeast cells are provided glucose without oxygen. ...
Chapter Nine - The Krebs Cycle
... – Overall reaction – conversion of succinate to fumarate – Only membrane in Krebs cycle that is membrane bound – Ubiquinone is used as a redox cofactor – – Metabolically irreversible • No known regulators ...
... – Overall reaction – conversion of succinate to fumarate – Only membrane in Krebs cycle that is membrane bound – Ubiquinone is used as a redox cofactor – – Metabolically irreversible • No known regulators ...
Chapter 3: Energy, Catalysis, and Biosynthesis
... for the reaction X→Y. The solid line in the energy diagram represents changes in energy as the product is converted to reactant under standard conditions. The dashed line shows changes observed when the same reaction takes place in the presence of a dedicated enzyme. Which equation below indicates h ...
... for the reaction X→Y. The solid line in the energy diagram represents changes in energy as the product is converted to reactant under standard conditions. The dashed line shows changes observed when the same reaction takes place in the presence of a dedicated enzyme. Which equation below indicates h ...
Section 2 The Calvin Cycle - Sonoma Valley High School
... the Calvin cycle are converted to a five-carbon sugar (RuBP) to keep the Calvin cycle operating. But some of the three-carbon sugars leave the Calvin cycle and are used to make organic compounds, in which energy is stored for later use. Chapter menu ...
... the Calvin cycle are converted to a five-carbon sugar (RuBP) to keep the Calvin cycle operating. But some of the three-carbon sugars leave the Calvin cycle and are used to make organic compounds, in which energy is stored for later use. Chapter menu ...
5. CHAPTER XI PHOTOSYNTHESIS
... • Next is the regeneration of the beginning substrate, ribulose 1,5-bisphosphate. It is known as the photosynthetic carbon reduction cycle, or Calvin cycle. • A totally new molecule of glyceraldehyde 3-phosphate can be spun off with every three revolutions of the cycle. • The new glyceraldehyde 3-ph ...
... • Next is the regeneration of the beginning substrate, ribulose 1,5-bisphosphate. It is known as the photosynthetic carbon reduction cycle, or Calvin cycle. • A totally new molecule of glyceraldehyde 3-phosphate can be spun off with every three revolutions of the cycle. • The new glyceraldehyde 3-ph ...
Document
... An Accounting of ATP Production by Cellular Respiration • During cellular respiration, most energy flows in this sequence: glucose NADH electron transport chain proton-motive force ATP • About 34% of the energy in a glucose molecule is transferred to ATP during cellular respiration, making ...
... An Accounting of ATP Production by Cellular Respiration • During cellular respiration, most energy flows in this sequence: glucose NADH electron transport chain proton-motive force ATP • About 34% of the energy in a glucose molecule is transferred to ATP during cellular respiration, making ...