Regio- and Enantioselective Alkane Hydroxylation with Engineered Cytochromes P450 BM-3 Peter Meinhold,
... at their ω-1, ω-2, and ω-3 positions using atmospheric dioxygen and nicotinamide adenine dinucleotide phosphate (NADPH) (Figure 1).24 Substrate is bound and hydroxylated in a hydrophobic pocket positioned directly above the heme cofactor in the P450 heme, or hydroxylase, domain. A peptide linker con ...
... at their ω-1, ω-2, and ω-3 positions using atmospheric dioxygen and nicotinamide adenine dinucleotide phosphate (NADPH) (Figure 1).24 Substrate is bound and hydroxylated in a hydrophobic pocket positioned directly above the heme cofactor in the P450 heme, or hydroxylase, domain. A peptide linker con ...
H. Heldt
... photosystem II and photosystem I 92 Iron atoms in cytochromes and in iron-sulfur centers have a central function as redox carriers 92 The electron transport by the cytochrome-b6/f complex is coupled to a proton transport 95 The number of protons pumped through the cyt-b6/f complex can be doubled by ...
... photosystem II and photosystem I 92 Iron atoms in cytochromes and in iron-sulfur centers have a central function as redox carriers 92 The electron transport by the cytochrome-b6/f complex is coupled to a proton transport 95 The number of protons pumped through the cyt-b6/f complex can be doubled by ...
Plant Biochemistry
... photosystem II and photosystem I 92 Iron atoms in cytochromes and in iron-sulfur centers have a central function as redox carriers 92 The electron transport by the cytochrome-b6/f complex is coupled to a proton transport 95 The number of protons pumped through the cyt-b6/f complex can be doubled by ...
... photosystem II and photosystem I 92 Iron atoms in cytochromes and in iron-sulfur centers have a central function as redox carriers 92 The electron transport by the cytochrome-b6/f complex is coupled to a proton transport 95 The number of protons pumped through the cyt-b6/f complex can be doubled by ...
H - IS MU
... 2 Carnitine carries long-chain activated fatty acids into the mitochondrial matrix Acyl-CoA itself cannot cross the inner mitochondrial membrane; instead, acyl groups are transferred to carnitine, transported across the membrane as acylcarnitine, and transferred back to CoA within the mitochondrial ...
... 2 Carnitine carries long-chain activated fatty acids into the mitochondrial matrix Acyl-CoA itself cannot cross the inner mitochondrial membrane; instead, acyl groups are transferred to carnitine, transported across the membrane as acylcarnitine, and transferred back to CoA within the mitochondrial ...
CHAPTER 6
... Figure 22.3 A mechanism for the pyruvate carboxylase reaction. Bicarbonate must be activated for attack by the pyruvate carbanion. This activation is driven by ATP and involves formation of a carbonylphosphate intermediate—a mixed anhydride of carbonic and phosphoric acids. (Carbonylphosphate and ca ...
... Figure 22.3 A mechanism for the pyruvate carboxylase reaction. Bicarbonate must be activated for attack by the pyruvate carbanion. This activation is driven by ATP and involves formation of a carbonylphosphate intermediate—a mixed anhydride of carbonic and phosphoric acids. (Carbonylphosphate and ca ...
ADP
... To produce NADPH (1) NADPH is the donor of hydrogen for various anabolic metabolism in organism (2) NADPH can participate in the hydroxylation reaction, involving biosynthesis or biotransformation in organism ...
... To produce NADPH (1) NADPH is the donor of hydrogen for various anabolic metabolism in organism (2) NADPH can participate in the hydroxylation reaction, involving biosynthesis or biotransformation in organism ...
Photosynthesis lab AP 2012
... Photosynthesis fuels ecosystems and replenishes the Earth’s atmosphere with oxygen. Like all enzyme-driven reactions, the rate of photosynthesis can be measured by either the disappearance of substrate or the accumulation of product (or by-products). The general summary equation for photosynthesis i ...
... Photosynthesis fuels ecosystems and replenishes the Earth’s atmosphere with oxygen. Like all enzyme-driven reactions, the rate of photosynthesis can be measured by either the disappearance of substrate or the accumulation of product (or by-products). The general summary equation for photosynthesis i ...
Nuclear Genetic Defects of Mitochondrial ATP Synthase
... identification of affected nuclear genes encoding either structural proteins or biogenetic and regulatory factors of OXPHOS machinery – representing so called “direct and indirect hits” (Dimauro 2011). Today more than 150 nuclear genetic defects have already been associated with disorders of mitocho ...
... identification of affected nuclear genes encoding either structural proteins or biogenetic and regulatory factors of OXPHOS machinery – representing so called “direct and indirect hits” (Dimauro 2011). Today more than 150 nuclear genetic defects have already been associated with disorders of mitocho ...
Modular organization of cardiac energy metabolism: energy
... steady state (Goldbeter & Nicolis 1976, Dzeja & Terzic 2003, Qian 2006, De la Fuente et al. 2010, Ge & Qian 2013). According to these theories, compartmentalized metabolic processes are integrated by metabolic channelling via enzymatic complexes which can associate physically with cytoskeleton creat ...
... steady state (Goldbeter & Nicolis 1976, Dzeja & Terzic 2003, Qian 2006, De la Fuente et al. 2010, Ge & Qian 2013). According to these theories, compartmentalized metabolic processes are integrated by metabolic channelling via enzymatic complexes which can associate physically with cytoskeleton creat ...
Theoretical studies on pyridoxal 5’-phosphate- catalyzed reactions of biological relevance 2014
... The spontaneous reactions involving the Cα bonds of amino acids in aqueous solution are amongst the slowest biological processes, some of which exhibit half-lifes of as much as 1.1 billion years (Radzicka1996, Snider2000, Wolfenden2001). This may question the possibility of life formation when consi ...
... The spontaneous reactions involving the Cα bonds of amino acids in aqueous solution are amongst the slowest biological processes, some of which exhibit half-lifes of as much as 1.1 billion years (Radzicka1996, Snider2000, Wolfenden2001). This may question the possibility of life formation when consi ...
E. coli
... deprotonation of NH4+ by an Asp causes a flap (324-328) to close over active site ammonia attacks glutamyl-g-P forming tetrahedral intermediate Pi and a proton are lost The flap opens and glutamine leaves ...
... deprotonation of NH4+ by an Asp causes a flap (324-328) to close over active site ammonia attacks glutamyl-g-P forming tetrahedral intermediate Pi and a proton are lost The flap opens and glutamine leaves ...
10 Translocation in the Phloem Chapter
... 10.2). In plants with secondary growth the phloem constitutes the inner bark. The cells of the phloem that conduct sugars and other organic materials throughout the plant are called sieve elements. Sieve element is a comprehensive term that includes both the highly differentiated sieve tube elements ...
... 10.2). In plants with secondary growth the phloem constitutes the inner bark. The cells of the phloem that conduct sugars and other organic materials throughout the plant are called sieve elements. Sieve element is a comprehensive term that includes both the highly differentiated sieve tube elements ...
Problem-Set Solutions
... as lactate, glycerol and certain amino acids) takes place in the liver. 24.66 maintain normal glucose blood levels in times of inadequate dietary carbohydrate intake 24.67 The last step of glycolysis is the conversion of the high-energy compound phosphoenolpyruvate to pyruvate. To reverse this step ...
... as lactate, glycerol and certain amino acids) takes place in the liver. 24.66 maintain normal glucose blood levels in times of inadequate dietary carbohydrate intake 24.67 The last step of glycolysis is the conversion of the high-energy compound phosphoenolpyruvate to pyruvate. To reverse this step ...
pyruvate dehydrogenase complex
... - Production of ATP increases until it matches rate of ATP consumption by energy-requiring reactions ...
... - Production of ATP increases until it matches rate of ATP consumption by energy-requiring reactions ...
Calculation of Biochemical Net Reactions and Pathways by Using
... The section on the mathematics of pathways describes three levels of thermodynamic treatment. The previous section was at Level 2, and in this section Level 3 is used. The advantage of using Level 3 is that the number of reactants is reduced. In this case ATP, ADP, Pi, NADox , and NADred, are remove ...
... The section on the mathematics of pathways describes three levels of thermodynamic treatment. The previous section was at Level 2, and in this section Level 3 is used. The advantage of using Level 3 is that the number of reactants is reduced. In this case ATP, ADP, Pi, NADox , and NADred, are remove ...
Secondary chlorophyll a luminescence decay kinetics from green
... *The competition for excitation energy from pathways other than light emission. The first two of these parameters form the substrates for luminescence. They are independent of each other [8] and therefore to some extent exchangeable with each other, i.e. the same intensity of luminescence may arise ...
... *The competition for excitation energy from pathways other than light emission. The first two of these parameters form the substrates for luminescence. They are independent of each other [8] and therefore to some extent exchangeable with each other, i.e. the same intensity of luminescence may arise ...
Phosphorylation of the F1Fo ATP Synthase Я Subunit
... striking mutant was the T262 site, for which the phosphomimetic (T262E) abolished activity, whereas the nonphosphorylatable strain (T262A) had an ATPase rate equivalent to wild type. Although T262E, like all of the  subunit mutants, was able to form the intact complex (F1Fo), this strain lacked a f ...
... striking mutant was the T262 site, for which the phosphomimetic (T262E) abolished activity, whereas the nonphosphorylatable strain (T262A) had an ATPase rate equivalent to wild type. Although T262E, like all of the  subunit mutants, was able to form the intact complex (F1Fo), this strain lacked a f ...
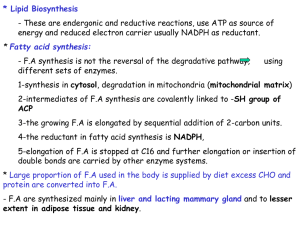
13synthesis
... 4-the reductant in fatty acid synthesis is NADPH, 5-elongation of F.A is stopped at C16 and further elongation or insertion of double bonds are carried by other enzyme systems. * Large proportion of F.A used in the body is supplied by diet excess CHO and protein are converted into F.A. - F.A are syn ...
... 4-the reductant in fatty acid synthesis is NADPH, 5-elongation of F.A is stopped at C16 and further elongation or insertion of double bonds are carried by other enzyme systems. * Large proportion of F.A used in the body is supplied by diet excess CHO and protein are converted into F.A. - F.A are syn ...
Leaf orientation, photorespiration and xanthophyll cycle protect
... During leaf development, the newly initiating leaves are often exposed to full sunlight at the topmost canopy, indicating that those young leaves have to endure extremely high irradiance. However, young leaves have lower photosynthesis activity per unit area compared with fully developed leaves (Kra ...
... During leaf development, the newly initiating leaves are often exposed to full sunlight at the topmost canopy, indicating that those young leaves have to endure extremely high irradiance. However, young leaves have lower photosynthesis activity per unit area compared with fully developed leaves (Kra ...
Theoretical Approaches to the Evolutionary Optimization of Glycolysis
... (a) Anaerobiosis. The whole process cannot depend on any external electron source or sink; redox reactions can of course occur but, in the global process, the initial substrate must have the same global oxidation degree as the end product. (b) Exergonism. On the basis of a given reasonable set of ex ...
... (a) Anaerobiosis. The whole process cannot depend on any external electron source or sink; redox reactions can of course occur but, in the global process, the initial substrate must have the same global oxidation degree as the end product. (b) Exergonism. On the basis of a given reasonable set of ex ...
Studying fast dynamics in biological complexes
... unprecedented throughput and time resolution. In the second part, picosecond time-resolved fluorescence spectroscopy was used to study ultrafast processes in photosynthesis such as excitation energy transfer and non-photochemical quenching (NPQ) on the ensemble level. Photosynthesis is a process in ...
... unprecedented throughput and time resolution. In the second part, picosecond time-resolved fluorescence spectroscopy was used to study ultrafast processes in photosynthesis such as excitation energy transfer and non-photochemical quenching (NPQ) on the ensemble level. Photosynthesis is a process in ...
The contributions of James Franck to
... a mechanism for energy migration, to explain the pioneering research of Emerson and Arnold (1932a, b) where it was shown that 2500 chlorophyll molecules cooperated to evolve one oxygen molecule. (3) Franck tried to explain how a natural system that had two independent types of functioning photosynth ...
... a mechanism for energy migration, to explain the pioneering research of Emerson and Arnold (1932a, b) where it was shown that 2500 chlorophyll molecules cooperated to evolve one oxygen molecule. (3) Franck tried to explain how a natural system that had two independent types of functioning photosynth ...
Document
... G-6-P dehydrogenase: Rate limiting step, controlled by NADP+ levels Glucose-6-phosphate Dehydrogenase catalyzes oxidation of the aldehyde (hemiacetal), at C1 of glucose-6-phosphate, to a carboxylic acid, in ester ...
... G-6-P dehydrogenase: Rate limiting step, controlled by NADP+ levels Glucose-6-phosphate Dehydrogenase catalyzes oxidation of the aldehyde (hemiacetal), at C1 of glucose-6-phosphate, to a carboxylic acid, in ester ...
Harvard University General Chemistry Practice Problems “The
... Ozone (O3) can be prepared in the laboratory by passing an electrical discharge through a quantity of oxygen gas (O2): 3 O2 (g) → 2 O 3 (g) An evacuated steel vessel with a volume of 10.00 liters is filled with 32.00 atm of pure O2 at 25°C. An electric discharge is passed through the vessel, causing ...
... Ozone (O3) can be prepared in the laboratory by passing an electrical discharge through a quantity of oxygen gas (O2): 3 O2 (g) → 2 O 3 (g) An evacuated steel vessel with a volume of 10.00 liters is filled with 32.00 atm of pure O2 at 25°C. An electric discharge is passed through the vessel, causing ...