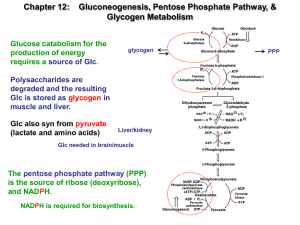
Biosynthesis of glucose – gluconeogenesis
... malate + NAD+ oxaloacetate + NADH2 Cytoplasmic oxaloacetate is decarboxylated to phosphoenolpyruvate by phosphoenolpyruvate carboxykinase ...
... malate + NAD+ oxaloacetate + NADH2 Cytoplasmic oxaloacetate is decarboxylated to phosphoenolpyruvate by phosphoenolpyruvate carboxykinase ...
Biology: Concepts and Connections, 6e (Campbell)
... C) often have "kinks" in their tails caused by the presence of a double bond between carbons. D) remain fluid because they are tightly packed against one another. E) form impermeable layers for cells . Answer: C الكولسترول المرتبط بأغشية الخاليا الحيوانية ...
... C) often have "kinks" in their tails caused by the presence of a double bond between carbons. D) remain fluid because they are tightly packed against one another. E) form impermeable layers for cells . Answer: C الكولسترول المرتبط بأغشية الخاليا الحيوانية ...
Carbohydrate Metabolism
... converts it to pyruvate. In the absence of oxygen, pyruvate is converted to lactate. When oxygen is present, pyruvate is further degraded to form acetyl-CoA. Significant amounts of energy in the form of ATP can be extracted from acetyl-CoA by the citric acid cycle and the electron transport system. ...
... converts it to pyruvate. In the absence of oxygen, pyruvate is converted to lactate. When oxygen is present, pyruvate is further degraded to form acetyl-CoA. Significant amounts of energy in the form of ATP can be extracted from acetyl-CoA by the citric acid cycle and the electron transport system. ...
COPYRIGHTED MATERIAL
... Biological treatment processes are the most important unit operations in wastewater treatment. Methods of purification in biological treatment units are similar to the selfpurification process that occurs naturally in rivers and streams, and involve many of the same microorganisms. Removal of organi ...
... Biological treatment processes are the most important unit operations in wastewater treatment. Methods of purification in biological treatment units are similar to the selfpurification process that occurs naturally in rivers and streams, and involve many of the same microorganisms. Removal of organi ...
the spectral contribution of carotenoids to light absorption and
... studies show that carotenoids play a key role in adaptation of plants to light stress and other unfavorable ecological conditions. Some reflectance indexes for measuring carotenoids content from reflectance spectra have been reported (Chappelle et al., 1992, Datt 1998). There are two main obstacles ...
... studies show that carotenoids play a key role in adaptation of plants to light stress and other unfavorable ecological conditions. Some reflectance indexes for measuring carotenoids content from reflectance spectra have been reported (Chappelle et al., 1992, Datt 1998). There are two main obstacles ...
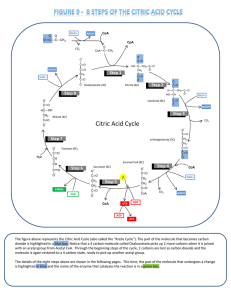
Citric Acid Cycle - BYU
... dioxide is highlighted in a blue box. Notice that a 4 carbon molecule called Oxaloacetate picks up 2 more carbons when it is joined with an acetyl group from Acetyl CoA. Through the beginning steps of the cycle, 2 carbons are lost as carbon dioxide and the molecule is again restored to a 4 carbon ...
... dioxide is highlighted in a blue box. Notice that a 4 carbon molecule called Oxaloacetate picks up 2 more carbons when it is joined with an acetyl group from Acetyl CoA. Through the beginning steps of the cycle, 2 carbons are lost as carbon dioxide and the molecule is again restored to a 4 carbon ...
ATP regulation in bioproduction
... of ATP-generating phosphoenolpyruvate carboxykinase (Pck) from Actinobacillus succinogenes in a mutant strain of Escherichia coli effectively enhances cell growth and succinic acid production [27] (Fig. 3). Further, succinic acid production by Enterobacter aerogenes is enhanced using a similar stra ...
... of ATP-generating phosphoenolpyruvate carboxykinase (Pck) from Actinobacillus succinogenes in a mutant strain of Escherichia coli effectively enhances cell growth and succinic acid production [27] (Fig. 3). Further, succinic acid production by Enterobacter aerogenes is enhanced using a similar stra ...
ATP regulation in bioproduction
... of ATP-generating phosphoenolpyruvate carboxykinase (Pck) from Actinobacillus succinogenes in a mutant strain of Escherichia coli effectively enhances cell growth and succinic acid production [27] (Fig. 3). Further, succinic acid production by Enterobacter aerogenes is enhanced using a similar stra ...
... of ATP-generating phosphoenolpyruvate carboxykinase (Pck) from Actinobacillus succinogenes in a mutant strain of Escherichia coli effectively enhances cell growth and succinic acid production [27] (Fig. 3). Further, succinic acid production by Enterobacter aerogenes is enhanced using a similar stra ...
Glycolysis and Gluconeogenesis
... Control of Glycolysis Of what value is glycolysis for cells? provides energy in form of ATP provides building blocks for synthetic reactions Where are most control points found? enzymes that catalyze irreversible reactions ...
... Control of Glycolysis Of what value is glycolysis for cells? provides energy in form of ATP provides building blocks for synthetic reactions Where are most control points found? enzymes that catalyze irreversible reactions ...
Bio 226: Cell and Molecular Biology
... Nutrient assimilation Assimilating N and S is very expensive! • Reducing NO3- to NH4+ costs 8 e- (1 NADPH + 6 Fd) • Assimilating NH4+ into amino acids also costs ATP + e• Nitrogen fixation costs 16 ATP + 8 e• SO42- reduction to S2- costs 8 e- + 2ATP • S2- assimilation into Cysteine costs 2 more e• ...
... Nutrient assimilation Assimilating N and S is very expensive! • Reducing NO3- to NH4+ costs 8 e- (1 NADPH + 6 Fd) • Assimilating NH4+ into amino acids also costs ATP + e• Nitrogen fixation costs 16 ATP + 8 e• SO42- reduction to S2- costs 8 e- + 2ATP • S2- assimilation into Cysteine costs 2 more e• ...
21. glycolysis
... with the intervention of molecular oxygen. This pathway is followed by aerobic animal and plant cells. (b) If the supply of oxygen is insufficient, as in vigorously contracting skeletal muscles, the pyruvate cannot be oxidized further for lack of oxygen. Under such conditions, it is then reduced to ...
... with the intervention of molecular oxygen. This pathway is followed by aerobic animal and plant cells. (b) If the supply of oxygen is insufficient, as in vigorously contracting skeletal muscles, the pyruvate cannot be oxidized further for lack of oxygen. Under such conditions, it is then reduced to ...
FREE Sample Here
... 26. (p. 41) Blood has a pH ranging from 7.35 to 7.45. Slight deviations from this can cause major problems or even death. You are doing an intense workout, and your skeletal muscle cells are producing metabolic acids such as lactic acid. Your blood pH does not drop significantly in spite of the meta ...
... 26. (p. 41) Blood has a pH ranging from 7.35 to 7.45. Slight deviations from this can cause major problems or even death. You are doing an intense workout, and your skeletal muscle cells are producing metabolic acids such as lactic acid. Your blood pH does not drop significantly in spite of the meta ...
Crystal structure of ATP sulfurylase from Saccharomyces cerevisiae
... Domain I is built by the N-terminal part of the peptide chain (residues 2±167) and comprises as a main motif a b-barrel, which is formed by ®ve antiparallel strands in a 1-3-4-5-2 topology. It is topologically similar to the pyruvate kinase b-barrel domain (Figure 3A), but in contrast to the pyruvat ...
... Domain I is built by the N-terminal part of the peptide chain (residues 2±167) and comprises as a main motif a b-barrel, which is formed by ®ve antiparallel strands in a 1-3-4-5-2 topology. It is topologically similar to the pyruvate kinase b-barrel domain (Figure 3A), but in contrast to the pyruvat ...
Chapter 6 Identifying and Measuring Transmembrane Helix–Helix
... contribution of nonspecific FRET, arising from random acceptor– donor colocalization in the bilayer (19–23). It also decreases the possibility of nonspecific aggregation that may result from hydrophobic fluorescent probes, and allows the monitoring of homodimerization with pyrene, which forms excite ...
... contribution of nonspecific FRET, arising from random acceptor– donor colocalization in the bilayer (19–23). It also decreases the possibility of nonspecific aggregation that may result from hydrophobic fluorescent probes, and allows the monitoring of homodimerization with pyrene, which forms excite ...
A new simple fluorimetric method to assay cytosolic ATP content
... NADPH fluorescence formed via HK/G6PDH coupled reactions as reported above (protocol IV). DWM isolation and measurements of PmitoKATP and PUCP activity by means of ∆Ψ and swelling experiments About 50–60 g of etiolated early seedlings were removed from seeds and mitochondria were isolated as reported ...
... NADPH fluorescence formed via HK/G6PDH coupled reactions as reported above (protocol IV). DWM isolation and measurements of PmitoKATP and PUCP activity by means of ∆Ψ and swelling experiments About 50–60 g of etiolated early seedlings were removed from seeds and mitochondria were isolated as reported ...
Chapter 5: Gases - HCC Learning Web
... 7. An exothermic reaction causes the surroundings to A) warm up. D) decrease its temperature. B) become acidic. E) release CO2. C) expand. Ans: A Category: Easy Section: 6.2 8. Copper metal has a specific heat of 0.385 J/g·°C. Calculate the amount of heat required to raise the temperature of 22.8 g ...
... 7. An exothermic reaction causes the surroundings to A) warm up. D) decrease its temperature. B) become acidic. E) release CO2. C) expand. Ans: A Category: Easy Section: 6.2 8. Copper metal has a specific heat of 0.385 J/g·°C. Calculate the amount of heat required to raise the temperature of 22.8 g ...
Homework 5-7 answers
... 7. An exothermic reaction causes the surroundings to A) warm up. D) decrease its temperature. B) become acidic. E) release CO2. C) expand. Ans: A Category: Easy Section: 6.2 8. Copper metal has a specific heat of 0.385 J/g·°C. Calculate the amount of heat required to raise the temperature of 22.8 g ...
... 7. An exothermic reaction causes the surroundings to A) warm up. D) decrease its temperature. B) become acidic. E) release CO2. C) expand. Ans: A Category: Easy Section: 6.2 8. Copper metal has a specific heat of 0.385 J/g·°C. Calculate the amount of heat required to raise the temperature of 22.8 g ...
Homework 5-8 answers
... A) the energy stored within the structural units of chemical substances. B) the energy associated with the random motion of atoms and molecules. C) solar energy, i.e. energy that comes from the sun. D) energy available by virtue of an object's position. Ans: C Category: Easy Section: 6.1 2. Thermal ...
... A) the energy stored within the structural units of chemical substances. B) the energy associated with the random motion of atoms and molecules. C) solar energy, i.e. energy that comes from the sun. D) energy available by virtue of an object's position. Ans: C Category: Easy Section: 6.1 2. Thermal ...
Energy systems and interplay of energy systems
... equivalent to 50–75 kilograms of ATP (see figure 5.3). The limited stores of ATP within the body are used up within about 1–2 seconds of maximal intensity activity. If it were not for the fact that ATP stores are constantly replenished during activity, muscular contraction could not continue. Once i ...
... equivalent to 50–75 kilograms of ATP (see figure 5.3). The limited stores of ATP within the body are used up within about 1–2 seconds of maximal intensity activity. If it were not for the fact that ATP stores are constantly replenished during activity, muscular contraction could not continue. Once i ...
uncorrected page proofs
... equivalent to 50–75 kilograms of ATP (see figure 5.3). The limited stores of ATP within the body are used up within about 1–2 seconds of maximal intensity activity. If it were not for the fact that ATP stores are constantly replenished during activity, muscular contraction could not continue. Once i ...
... equivalent to 50–75 kilograms of ATP (see figure 5.3). The limited stores of ATP within the body are used up within about 1–2 seconds of maximal intensity activity. If it were not for the fact that ATP stores are constantly replenished during activity, muscular contraction could not continue. Once i ...
Unit F214
... areas such as IT, business, languages, teaching/training, administration and secretarial skills. It is also responsible for developing new specifications to meet national requirements and the needs of students and teachers. OCR is a not-for-profit organisation; any surplus made is invested back into ...
... areas such as IT, business, languages, teaching/training, administration and secretarial skills. It is also responsible for developing new specifications to meet national requirements and the needs of students and teachers. OCR is a not-for-profit organisation; any surplus made is invested back into ...
Document
... Fate of Pyruvate • Two anerobic pathways: (Low O2 ) - to lactate via lactate dehydrogenase in muscle - to ethanol (fermentation) via ethanol dehydrogenase • Aerobic pathway – through citric acid cycle and respiration; Enough O2,this pathway yields far more ...
... Fate of Pyruvate • Two anerobic pathways: (Low O2 ) - to lactate via lactate dehydrogenase in muscle - to ethanol (fermentation) via ethanol dehydrogenase • Aerobic pathway – through citric acid cycle and respiration; Enough O2,this pathway yields far more ...
Probing the origins of glutathione biosynthesis through biochemical
... [35]; however, biochemical analysis of these proteins has not been performed. The nucleotide sequence of Synechocystis gshA (1170 bp) encodes a 389 amino acid protein with a predicted molecular mass of 44.1 kDa and a pI value of 5.35 (Figure 2). Amino acid sequence comparisons show that it shares 64 ...
... [35]; however, biochemical analysis of these proteins has not been performed. The nucleotide sequence of Synechocystis gshA (1170 bp) encodes a 389 amino acid protein with a predicted molecular mass of 44.1 kDa and a pI value of 5.35 (Figure 2). Amino acid sequence comparisons show that it shares 64 ...
Design and analysis of metabolic pathways supporting
... of reducing power; (v) low reduction potential, such that a direct reduction of NAD(P)H is feasible (reduction potential b −300 mV); (vi) availability of an enzymatic apparatus capable of catalyzing electron transfer from the donor to NAD(P)H and (vii) fast catalysis of electron transfer from the do ...
... of reducing power; (v) low reduction potential, such that a direct reduction of NAD(P)H is feasible (reduction potential b −300 mV); (vi) availability of an enzymatic apparatus capable of catalyzing electron transfer from the donor to NAD(P)H and (vii) fast catalysis of electron transfer from the do ...