electron transport chain
... • In lactic acid fermentation, pyruvate is reduced to NADH, forming lactate as an end product, with no release of CO2 • Lactic acid fermentation by some fungi and bacteria is used to make cheese and yogurt • Human muscle cells use lactic acid fermentation to generate ATP when O2 is scarce ...
... • In lactic acid fermentation, pyruvate is reduced to NADH, forming lactate as an end product, with no release of CO2 • Lactic acid fermentation by some fungi and bacteria is used to make cheese and yogurt • Human muscle cells use lactic acid fermentation to generate ATP when O2 is scarce ...
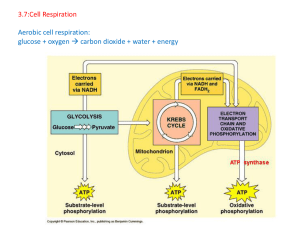
3.7:Cell Respiration Aerobic cell respiration: glucose
... IB Question: Compare anaerobic cellular respiration and aerobic cellular respiration. [5] Direct comparisons must be made to achieve a mark. anaerobic in the absence of oxygen whereas aerobic in the presence of oxygen; both may produce 2 CO ; both produce ATP; aerobic releases considerably more ATP ...
... IB Question: Compare anaerobic cellular respiration and aerobic cellular respiration. [5] Direct comparisons must be made to achieve a mark. anaerobic in the absence of oxygen whereas aerobic in the presence of oxygen; both may produce 2 CO ; both produce ATP; aerobic releases considerably more ATP ...
Cellular Respiration
... to NADH, forming lactate as an end product, with no release of CO2 Lactic acid fermentation by some fungi and bacteria is used to make cheese and yogurt Human muscle cells use lactic acid fermentation to generate ATP when O2 is scarce ...
... to NADH, forming lactate as an end product, with no release of CO2 Lactic acid fermentation by some fungi and bacteria is used to make cheese and yogurt Human muscle cells use lactic acid fermentation to generate ATP when O2 is scarce ...
8 Cellular Respiration-An Overview
... Glucose, or any carbon-based molecule, can be burned in oxygen (oxidized) to produce carbon dioxide and water. Combustion reactions release large amounts of energy. However, the energy release is uncontrolled. An organism would not be able to handle all that energy at once to do the work of the cell ...
... Glucose, or any carbon-based molecule, can be burned in oxygen (oxidized) to produce carbon dioxide and water. Combustion reactions release large amounts of energy. However, the energy release is uncontrolled. An organism would not be able to handle all that energy at once to do the work of the cell ...
Atoms and Molecules
... general review of first-year chemistry material during the first one or two class meetings (typically the first week of school). We have a quiz on every Friday. So when we meet on a Friday we will have our first quiz. We will then immediately begin chapter 3. So that must be read prior to the second ...
... general review of first-year chemistry material during the first one or two class meetings (typically the first week of school). We have a quiz on every Friday. So when we meet on a Friday we will have our first quiz. We will then immediately begin chapter 3. So that must be read prior to the second ...
How much glucose does a plant make?
... Light is needed for photosynthesis to occur. The plant’s leaves use the light to make a sugar called glucose. ...
... Light is needed for photosynthesis to occur. The plant’s leaves use the light to make a sugar called glucose. ...
ATP
... • Acetyl CoA carries acetyl groups, 2carbon remnants of the nutrients • Acetyl CoA enters the citric acid cycle – Electrons and hydrogen atoms are harvested – Acetyl group is oxidized to produce CO2 – Electrons and hydrogen atoms harvested are used to produce ATP during oxidative phosphorylation ...
... • Acetyl CoA carries acetyl groups, 2carbon remnants of the nutrients • Acetyl CoA enters the citric acid cycle – Electrons and hydrogen atoms are harvested – Acetyl group is oxidized to produce CO2 – Electrons and hydrogen atoms harvested are used to produce ATP during oxidative phosphorylation ...
Relationship between Photosynthesis and Cellular Respiration
... H+ ions must move back from a higher lower concentration Only return to inner compartment through ATP synthases, “gates of the dam” As they move through, activate ATP synthase to make ATP from ADP + Pi This process is called Chemiosmosis (ATP production linked to H+ gradient) ...
... H+ ions must move back from a higher lower concentration Only return to inner compartment through ATP synthases, “gates of the dam” As they move through, activate ATP synthase to make ATP from ADP + Pi This process is called Chemiosmosis (ATP production linked to H+ gradient) ...
Cellular Energy
... compounds to form twelve 3-carbon molecules called 3-PGA. The chemical energy stored in ATP and NADPH is transferred to the 3-PGA molecules to form high-energy molecules called G3P. ...
... compounds to form twelve 3-carbon molecules called 3-PGA. The chemical energy stored in ATP and NADPH is transferred to the 3-PGA molecules to form high-energy molecules called G3P. ...
Exam 1 Q2 Review Sheet
... a. Starting with food on your kitchen table and oxygen in the air, explain how cells convert the stored chemical potential “energy” in food to stored chemical potential “energy” in ATP. Draw a diagram to accompany your explanation if you wish. Be as specific as possible. Make sure you discuss affini ...
... a. Starting with food on your kitchen table and oxygen in the air, explain how cells convert the stored chemical potential “energy” in food to stored chemical potential “energy” in ATP. Draw a diagram to accompany your explanation if you wish. Be as specific as possible. Make sure you discuss affini ...
Glycolysis & Fermentation
... 5 Steps in Krebs cycle Step 1 – produces citric acid Step 2 – releases CO2 Step 3 – releases CO2 Step 4 – conversion of 4-carbon compound Step 5 – 4-carbon compound converted back to oxaloacetic acid ...
... 5 Steps in Krebs cycle Step 1 – produces citric acid Step 2 – releases CO2 Step 3 – releases CO2 Step 4 – conversion of 4-carbon compound Step 5 – 4-carbon compound converted back to oxaloacetic acid ...
1. The molecule that is most directly used to power different cell
... ATP stands for adenosine triphosphate. The tri in the name tells you that it has a 3 phosphate group tail. The triphosphate tail is an important part of the molecule because it store energy in this high energy bond. ...
... ATP stands for adenosine triphosphate. The tri in the name tells you that it has a 3 phosphate group tail. The triphosphate tail is an important part of the molecule because it store energy in this high energy bond. ...
PG1005 Lecture 12 Kreb`s Citric Acid Cycle
... • For each molecule of glucose a net production of 2 ATP occurs. ...
... • For each molecule of glucose a net production of 2 ATP occurs. ...
Honors Biology A 4W5 Respiration (divide by
... is the conversion of pyruvate into carbon dioxide and high energy electrons, also called the Krebs cycle, or the citric acid cycle. is the abbreviation for the enzyme that helps convert a 2 carbon molecule with a 4 carbon molecule to make citric acid. series of reactions taking place on the mitochon ...
... is the conversion of pyruvate into carbon dioxide and high energy electrons, also called the Krebs cycle, or the citric acid cycle. is the abbreviation for the enzyme that helps convert a 2 carbon molecule with a 4 carbon molecule to make citric acid. series of reactions taking place on the mitochon ...
LECTURE 18 - Budostuff
... Aerobic respiration = lots of energy C6H12O6 + 6 O2 6 CO2 + 6 H2O + ~38 ATP ...
... Aerobic respiration = lots of energy C6H12O6 + 6 O2 6 CO2 + 6 H2O + ~38 ATP ...
Slide 1
... Pyruvate transported to mitochondrion Pyruvate Dehydrogenase-decarboxylates pyruvate and oxidizes forming Acetyl-CoA, CO2, and NADH <== X 2 for each glucose Krebs Cycle oxidizes Acetyl-CoA producing 2 CO2, 3 NADH, FADH2, and GTP <== X 2 for each glucose NADH and FADH2 have high potential ene ...
... Pyruvate transported to mitochondrion Pyruvate Dehydrogenase-decarboxylates pyruvate and oxidizes forming Acetyl-CoA, CO2, and NADH <== X 2 for each glucose Krebs Cycle oxidizes Acetyl-CoA producing 2 CO2, 3 NADH, FADH2, and GTP <== X 2 for each glucose NADH and FADH2 have high potential ene ...
Chapter 7A- Cellular Respiration: Glycolysis - TJ
... 16. A substance produced during photosynthesis that is used for completion of cellular respiration is a. water. c. NADPH. b. ATP. d. oxygen. 17. When glycolysis occurs, a. a molecule of glucose is split. b. two molecules of pyruvic acid are made. c. some ATP is produced. d. All of the above ...
... 16. A substance produced during photosynthesis that is used for completion of cellular respiration is a. water. c. NADPH. b. ATP. d. oxygen. 17. When glycolysis occurs, a. a molecule of glucose is split. b. two molecules of pyruvic acid are made. c. some ATP is produced. d. All of the above ...
Chromatography lab - Castle High School
... (its absorption spectrum peaks at 700 nm), and PS II contains a specialized chlorophyll a molecule called P680 (its absorption spectrum peaks at 680 nm). When light is absorbed by leaf pigments, electrons within each photosystem are boosted to a higher energy level and the energy is captured in the ...
... (its absorption spectrum peaks at 700 nm), and PS II contains a specialized chlorophyll a molecule called P680 (its absorption spectrum peaks at 680 nm). When light is absorbed by leaf pigments, electrons within each photosystem are boosted to a higher energy level and the energy is captured in the ...
Cellular Respiration and Fermentation
... The purpose of fermentation reactions is a) To regenerate NAD+ so glycolysis can continue b) To make alcohol or lactic acid that cells can metabolize for energy under anaerobic conditions c) To make additional ATP when respiration can’t make ATP fast enough d) To slow down cellular oxygen consu ...
... The purpose of fermentation reactions is a) To regenerate NAD+ so glycolysis can continue b) To make alcohol or lactic acid that cells can metabolize for energy under anaerobic conditions c) To make additional ATP when respiration can’t make ATP fast enough d) To slow down cellular oxygen consu ...
Electrons
... compounds. Each atom of carbon has four electrons in its outer energy level, which makes it possible for each carbon atom to form four bonds with other atoms. • As a result, carbon atoms can form long chains. A huge number of different carbon compounds exist. Each compound has a ...
... compounds. Each atom of carbon has four electrons in its outer energy level, which makes it possible for each carbon atom to form four bonds with other atoms. • As a result, carbon atoms can form long chains. A huge number of different carbon compounds exist. Each compound has a ...
Oxidation-Reduction Enzymes
... enthalpy or Gibbs energy G) than the original reacting substances, and the difference in energy content ∆G may appear as heat or it can be transformed to other kinds of useful energy, e.g. energy of chemical bonds in the reacting system. Many redox reactions in the cell are coupled with the formatio ...
... enthalpy or Gibbs energy G) than the original reacting substances, and the difference in energy content ∆G may appear as heat or it can be transformed to other kinds of useful energy, e.g. energy of chemical bonds in the reacting system. Many redox reactions in the cell are coupled with the formatio ...
O 2
... formula shows glucose, but that is just an example could be other sugars, fats or proteins ...
... formula shows glucose, but that is just an example could be other sugars, fats or proteins ...
Light
... • Because 12 molecules of water are consumed and 6 new molecules are formed, we can simplify the equation by indicating only the net consumption of water (This is the equation most familiar to you) 6 CO2 + 6 H2O + Light energy C6H12O6 + 6 O2 ...
... • Because 12 molecules of water are consumed and 6 new molecules are formed, we can simplify the equation by indicating only the net consumption of water (This is the equation most familiar to you) 6 CO2 + 6 H2O + Light energy C6H12O6 + 6 O2 ...
Pre-Test
... Which of the following would be unlikely to contribute to the substrate specificity of an enzyme? (Concept 8.4 ) [Hint] A similar shape exists between a pocket on the surface of the enzyme and a functional group on the substrate. ...
... Which of the following would be unlikely to contribute to the substrate specificity of an enzyme? (Concept 8.4 ) [Hint] A similar shape exists between a pocket on the surface of the enzyme and a functional group on the substrate. ...
Chapter 9 review sheet
... cells transfer the stored chemical potential “energy” in food to stored chemical potential “energy” in ATP. Draw a diagram to accompany your explanation if you wish. Be as specific as possible. Make sure you discuss affinity, reduction, oxidation, all of the energy transfers and when exergonic proce ...
... cells transfer the stored chemical potential “energy” in food to stored chemical potential “energy” in ATP. Draw a diagram to accompany your explanation if you wish. Be as specific as possible. Make sure you discuss affinity, reduction, oxidation, all of the energy transfers and when exergonic proce ...