faculty of sciences - Guru Nanak Dev University
... Developing habits of independent and fast reading : Students will be required to read a prescribed prose anthology titled Selections from Modern English Prose (Ed. Haladhar Panda published by University Press, Hyderabad). The essays in the anthology will be read by students at home with the help of ...
... Developing habits of independent and fast reading : Students will be required to read a prescribed prose anthology titled Selections from Modern English Prose (Ed. Haladhar Panda published by University Press, Hyderabad). The essays in the anthology will be read by students at home with the help of ...
lecture 11 catalysis_hydrogenation of alkenes
... oxidative addition of H2 to the 3‐coordinate d8 RhCl(PPh3)2 to give 5‐coordinate d6 RhH2Cl(PPh3)2 relative to the corresponding 4‐coordinate→6‐coordinate conversion is consistent with the tendency for faster reductive elimination from 5‐coordinate d6 species ...
... oxidative addition of H2 to the 3‐coordinate d8 RhCl(PPh3)2 to give 5‐coordinate d6 RhH2Cl(PPh3)2 relative to the corresponding 4‐coordinate→6‐coordinate conversion is consistent with the tendency for faster reductive elimination from 5‐coordinate d6 species ...
13 CHEMICAL EQUILIBRIUM W MODULE - 5
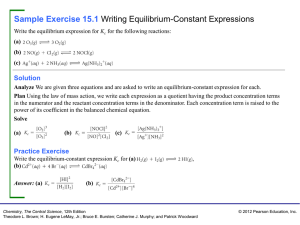
... reaction, it is believed that all the reactants would be converted into products with the release or absorption of energy. This is not true in all cases. Many chemical reactions proceed only to a certain extent and stop. When analysed, the resulting mixture contains both the reactants and products. ...
... reaction, it is believed that all the reactants would be converted into products with the release or absorption of energy. This is not true in all cases. Many chemical reactions proceed only to a certain extent and stop. When analysed, the resulting mixture contains both the reactants and products. ...
OCR A Level Chemistry A H432 Specification
... Our new A Level in Chemistry A builds on our existing popular course. We’ve based the redevelopment of our A level sciences on an understanding of what works well in centres large and small and have updated areas of content and assessment where stakeholders have identified that improvements could be ...
... Our new A Level in Chemistry A builds on our existing popular course. We’ve based the redevelopment of our A level sciences on an understanding of what works well in centres large and small and have updated areas of content and assessment where stakeholders have identified that improvements could be ...
Aromatic Compounds
... each of the n successive energy levels – a total of 4n + 2. Any other number would leave a bonding energy level partially unfilled Energy levels of the six benzene p molecular orbitals The lowest-energy MO, y1, occurs single and contains a pair of electrons y2 and y3, are degenerate, and it takes tw ...
... each of the n successive energy levels – a total of 4n + 2. Any other number would leave a bonding energy level partially unfilled Energy levels of the six benzene p molecular orbitals The lowest-energy MO, y1, occurs single and contains a pair of electrons y2 and y3, are degenerate, and it takes tw ...
www.xtremepapers.net
... deduce the numbers of protons, neutrons and electrons present in both atoms and ions given proton and nucleon numbers (and charge) ...
... deduce the numbers of protons, neutrons and electrons present in both atoms and ions given proton and nucleon numbers (and charge) ...
Answers - logo Pre-U Chemistry Textbook
... The two things that affect the size of hydration energies are ionic radius and the charge on the ion. The higher the charge on the ion the more exothermic ∆hydrH. The value for Mg2+ is nearly five times as large as Na+. Al3+ is nearly two and a half times as big as Mg2+. By comparing the values for ...
... The two things that affect the size of hydration energies are ionic radius and the charge on the ion. The higher the charge on the ion the more exothermic ∆hydrH. The value for Mg2+ is nearly five times as large as Na+. Al3+ is nearly two and a half times as big as Mg2+. By comparing the values for ...
The d-Block Elements
... example, the 4s23d10 electron configuration of zinc results in its strong tendency to form the stable Zn2+ ion, with a 3d10 electron configuration, whereas Cu+, which also has a 3d10 electron configuration, is the only stable monocation formed by a first-row transition metal. Similarly, with a half- ...
... example, the 4s23d10 electron configuration of zinc results in its strong tendency to form the stable Zn2+ ion, with a 3d10 electron configuration, whereas Cu+, which also has a 3d10 electron configuration, is the only stable monocation formed by a first-row transition metal. Similarly, with a half- ...
Monomer
... ⇒ reasons are not very clear but there is a trend. stereoselectivity and activity are dependent on the crystal structure, steric size, ligands, and the electronegativity of the transition metal. ...
... ⇒ reasons are not very clear but there is a trend. stereoselectivity and activity are dependent on the crystal structure, steric size, ligands, and the electronegativity of the transition metal. ...