HIGHLY SELECTIVE RHODIUM–CATALYZED C–H BORYLATIONS IN
... thanks to Alphonse for opening your heart…and your liquor cabinet. In addition to those mentioned above, thank you to everyone whom I crossed paths with during my time here at Queen’s. Particularly, shout out to Boniface, Bachus, Fraser, Fowler, Anthony and Gurpaul amongst many others who were there ...
... thanks to Alphonse for opening your heart…and your liquor cabinet. In addition to those mentioned above, thank you to everyone whom I crossed paths with during my time here at Queen’s. Particularly, shout out to Boniface, Bachus, Fraser, Fowler, Anthony and Gurpaul amongst many others who were there ...
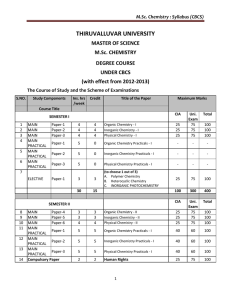
M.Sc. Chemistry : Syllabus (CBCS)
... Stability of complexes; thermodynamic aspects of complex formation; factors affecting stability, HSAB approach. Determination of stability constants by spectrophotometric, polarographic and potentiomteric methods. UNIT-IV: COORDINATION CHEMISTRY II Stereochemical aspects; Stereoisomerism in inorgani ...
... Stability of complexes; thermodynamic aspects of complex formation; factors affecting stability, HSAB approach. Determination of stability constants by spectrophotometric, polarographic and potentiomteric methods. UNIT-IV: COORDINATION CHEMISTRY II Stereochemical aspects; Stereoisomerism in inorgani ...
Chapter 4 C-C vs C-H Bond Oxidative Addition in PCX (X=P,N,O
... 7) (26). C-C bond activation takes place upon heating. Significantly, whereas CH activation of the two methyl groups is observed, only the C-C bond between the chelating arms is activated, demonstrating that the chelating methoxy ligand is both essential and sufficient for C-C activation in this sys ...
... 7) (26). C-C bond activation takes place upon heating. Significantly, whereas CH activation of the two methyl groups is observed, only the C-C bond between the chelating arms is activated, demonstrating that the chelating methoxy ligand is both essential and sufficient for C-C activation in this sys ...
NEW MONO- AND DINUCLEAR RUTHENIUM COMPLEXES CONTAINING THE 3,5-BIS(2- PYRIDYL)PYRAZOLE LIGAND. SYNTHESIS,
... synthesized and characterized by means of structural, spectroscopic and electrochemical techniques. These complexes have the formula [Ru2(µ-X)(bpp)(trpy)2]2+, where X = Cl, 1, and acetate, 2. The chloro and acetato bridges can be hydrolyzed in basic and acidic media, respectively, to generate the di ...
... synthesized and characterized by means of structural, spectroscopic and electrochemical techniques. These complexes have the formula [Ru2(µ-X)(bpp)(trpy)2]2+, where X = Cl, 1, and acetate, 2. The chloro and acetato bridges can be hydrolyzed in basic and acidic media, respectively, to generate the di ...
Unit 4 - Chemical Equilibrium
... _ _ _ _ _ _ _ _ _ _. This means the products can react together and turn back into the _ _ _ _ _ _ _ _ reactants. In other words, the reaction can go _ _ _ _ ways. When a reversible reaction is set up in a _ _ _ _ _ _ container, the forward reaction happens much faster than the reverse reaction at f ...
... _ _ _ _ _ _ _ _ _ _. This means the products can react together and turn back into the _ _ _ _ _ _ _ _ reactants. In other words, the reaction can go _ _ _ _ ways. When a reversible reaction is set up in a _ _ _ _ _ _ container, the forward reaction happens much faster than the reverse reaction at f ...
Problem 1-2 - IPN-Kiel
... detailed mechanism of the saponification of one of the three fatty acid residues. Explain why this equilibrium reaction drifts to the products. ...
... detailed mechanism of the saponification of one of the three fatty acid residues. Explain why this equilibrium reaction drifts to the products. ...
Test
... A 1-L container originally holds 0.4 mol of N2, 0.1 mol of O2, and 0.08 mole of NO. If the volume of the container holding the equilibrium mixture of N2, O2, and NO is decreased to 0.5 L without changing the quantities of the gases present, how will their concentrations change? a) The concentration ...
... A 1-L container originally holds 0.4 mol of N2, 0.1 mol of O2, and 0.08 mole of NO. If the volume of the container holding the equilibrium mixture of N2, O2, and NO is decreased to 0.5 L without changing the quantities of the gases present, how will their concentrations change? a) The concentration ...
dr.ebtehal Lec3
... catabolic metabolism of alcohol. It is commonly thought that the flush reaction is caused by an inability to metabolize alcohol. ...
... catabolic metabolism of alcohol. It is commonly thought that the flush reaction is caused by an inability to metabolize alcohol. ...
lec-3- 211( Elim+ Re..
... catabolic metabolism of alcohol. It is commonly thought that the flush reaction is caused by an inability to metabolize alcohol. ...
... catabolic metabolism of alcohol. It is commonly thought that the flush reaction is caused by an inability to metabolize alcohol. ...
Alkenes 3 - ChemWeb (UCC)
... This reaction illustrated above is called a 1,2- or -elimination to indicate that the groups being eliminated are located on adjacent atoms in the starting material as compared to a 1,1- or -elimination where both are located on the same carbon atom This, in itself, tells you nothing about the act ...
... This reaction illustrated above is called a 1,2- or -elimination to indicate that the groups being eliminated are located on adjacent atoms in the starting material as compared to a 1,1- or -elimination where both are located on the same carbon atom This, in itself, tells you nothing about the act ...
Problem Authors - PianetaChimica
... Kinetics: Integrated first order rate equation; analysis of complex reaction mechanisms using the steady state approximation; determination of reaction order and activation energy. Thermodynamics: Relationship between equilibrium constant, electromotive force and standard Gibbs free energy; the vari ...
... Kinetics: Integrated first order rate equation; analysis of complex reaction mechanisms using the steady state approximation; determination of reaction order and activation energy. Thermodynamics: Relationship between equilibrium constant, electromotive force and standard Gibbs free energy; the vari ...