Monomer
... ⇒ reasons are not very clear but there is a trend. stereoselectivity and activity are dependent on the crystal structure, steric size, ligands, and the electronegativity of the transition metal. ...
... ⇒ reasons are not very clear but there is a trend. stereoselectivity and activity are dependent on the crystal structure, steric size, ligands, and the electronegativity of the transition metal. ...
1412e3 - studylib.net
... according to the above reaction? The speed of light is 2.9979x108 m/s and 1 a.m.u =6.022x1023 g. 27. A student gave a molecule the following name; 3-methyl-4isopropylpentane. However, the professor pointed out that, although the molecule could be correctly drawn from this name, the name did not foll ...
... according to the above reaction? The speed of light is 2.9979x108 m/s and 1 a.m.u =6.022x1023 g. 27. A student gave a molecule the following name; 3-methyl-4isopropylpentane. However, the professor pointed out that, although the molecule could be correctly drawn from this name, the name did not foll ...
Preparation and Inner-sphere Oxidation of Ternary Iminodiacetato Chromium [III]
... some amino acids as secondary ligands, by NBS were studied. These reactions were found to proceed via a mechanism in which coordinated water was replaced by NBS prior to electron transfer step. Also, through bridging of NBS to the hydroxo conjugate species of the complex. Oxidation proceeds by homol ...
... some amino acids as secondary ligands, by NBS were studied. These reactions were found to proceed via a mechanism in which coordinated water was replaced by NBS prior to electron transfer step. Also, through bridging of NBS to the hydroxo conjugate species of the complex. Oxidation proceeds by homol ...
SMK RAJA PEREMPUAN, IPOH
... hydration, solution, neutralisation, atomisation, ionisation energy 4. explain the terms enthalphy change of reaction and standard conditions 5. calcualte enthalphy changes from experimental results ...
... hydration, solution, neutralisation, atomisation, ionisation energy 4. explain the terms enthalphy change of reaction and standard conditions 5. calcualte enthalphy changes from experimental results ...
Chapter 11
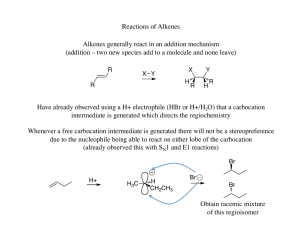
... The halonium ion thus directs both the regiochemistry (oxygen adds to the carbon that can best stabilize the partial positive charge) and the stereochemistry (due to the three membered ring the oxygen must add anti to the the bromine already present) ...
... The halonium ion thus directs both the regiochemistry (oxygen adds to the carbon that can best stabilize the partial positive charge) and the stereochemistry (due to the three membered ring the oxygen must add anti to the the bromine already present) ...
Exam 3 - Napa Valley College
... good he added twice as much benzaldehyde as Grignard reagent and got a lot of white crystalline product. When he analyzed his product he found that he had not made diphenyl methanol, but diphenyl methanal (also called benzophenone) instead. When he asked his research director about it he was told th ...
... good he added twice as much benzaldehyde as Grignard reagent and got a lot of white crystalline product. When he analyzed his product he found that he had not made diphenyl methanol, but diphenyl methanal (also called benzophenone) instead. When he asked his research director about it he was told th ...
Future perspectives in catalysis - NRSC
... bulk chemistry and petrochemistry. But shifting demands and new environmental challenges require new catalytic solutions. Changes in our energy economy have driven a growing demand for gas and coal, presenting new challenges for catalytic technology in areas such as liquefaction. In addition, cataly ...
... bulk chemistry and petrochemistry. But shifting demands and new environmental challenges require new catalytic solutions. Changes in our energy economy have driven a growing demand for gas and coal, presenting new challenges for catalytic technology in areas such as liquefaction. In addition, cataly ...
17: Oxidation and Reduction
... Things become more pOsitive when they are Oxidized. Similarly, both rEduction and nEgative have the same second letter "E" and things become more nEgative when they are rEduced. ...
... Things become more pOsitive when they are Oxidized. Similarly, both rEduction and nEgative have the same second letter "E" and things become more nEgative when they are rEduced. ...








![Preparation and Inner-sphere Oxidation of Ternary Iminodiacetato Chromium [III]](http://s1.studyres.com/store/data/008844767_1-9b02a033035d53dea970333df8a85c48-300x300.png)