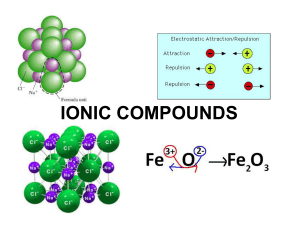
Chemistry - El Camino College
... _______ of electrons in their outer orbitals. Molecules with covalent bonds are represented 2 ways: a. ___________ formulas in which each pair of shared electrons is represented by a line (e.g.: O=C=O). b. __________ formulas that show only the number of each type of atom in the molecule (e.g.: CO2) ...
... _______ of electrons in their outer orbitals. Molecules with covalent bonds are represented 2 ways: a. ___________ formulas in which each pair of shared electrons is represented by a line (e.g.: O=C=O). b. __________ formulas that show only the number of each type of atom in the molecule (e.g.: CO2) ...
Chapter 32 * electrostatics
... from one and placed on the other, ie: charged Once an object is charged, touching something will cause some of the charge to transfer ...
... from one and placed on the other, ie: charged Once an object is charged, touching something will cause some of the charge to transfer ...
Chemical Stability
... Chemical Bonding • Chemical Bonding occurs to achieve Chemical Stability. • Atoms gain, lose or share valence electrons. Why do Atoms Bond? https://www.youtube.com/watch?v=JOL-nUt_vfo&feature=youtu.be&app=desktop ...
... Chemical Bonding • Chemical Bonding occurs to achieve Chemical Stability. • Atoms gain, lose or share valence electrons. Why do Atoms Bond? https://www.youtube.com/watch?v=JOL-nUt_vfo&feature=youtu.be&app=desktop ...
Preliminary Course Atomic Structure 1 + 2
... Sodium Neon (noble gas) has 10 electrons (Z=10). It is unreactive. Sodium (group 1 metal) has 11 electrons (Z=11) Therefore sodium desperately wants to lose an electron to gain a noble gas configuration ...
... Sodium Neon (noble gas) has 10 electrons (Z=10). It is unreactive. Sodium (group 1 metal) has 11 electrons (Z=11) Therefore sodium desperately wants to lose an electron to gain a noble gas configuration ...
Section 8.3 Names and Formulas of Ionic Compounds Formula Unit
... gaining electrons. • 3. Noble gases are relatively unreactive because they have eight electrone I n their outermost energy ...
... gaining electrons. • 3. Noble gases are relatively unreactive because they have eight electrone I n their outermost energy ...
SEMESTER 1 EXAM Prblms/Short Ans
... 4. A prospector finds 39.39 g of gold (Au), with a gram atomic mass = 196.9665g/mol. How many Au atoms does she ...
... 4. A prospector finds 39.39 g of gold (Au), with a gram atomic mass = 196.9665g/mol. How many Au atoms does she ...
Vocabulary: "Chemical Bonding"
... 23. What does a subscript represent in a chemical formula? 24. Why would scientists need to determine a compound’s empirical formula? ...
... 23. What does a subscript represent in a chemical formula? 24. Why would scientists need to determine a compound’s empirical formula? ...
Honors Midterm Review – 2015-16
... _________ responsible for the uncertainty principle which states that it is impossible to know (with any great degree of certainty) both the location and velocity of an electron) _________ responsible for the planetary model of the atom, where electrons traveled in distinct paths around the nucleus ...
... _________ responsible for the uncertainty principle which states that it is impossible to know (with any great degree of certainty) both the location and velocity of an electron) _________ responsible for the planetary model of the atom, where electrons traveled in distinct paths around the nucleus ...
Soil solution part 3
... • Ligands can be inorganic or organic • Analytical methods don’t differentiate between free and complexed ions: Mtotal = Mn+free + Mcomplexed ...
... • Ligands can be inorganic or organic • Analytical methods don’t differentiate between free and complexed ions: Mtotal = Mn+free + Mcomplexed ...
Bonding - Graham ISD
... because compounds of these atoms are almost always less stable than the original atom. Atoms with a partially stable outer energy level can lose, gain, or share electrons to obtain a stable outer energy level. ...
... because compounds of these atoms are almost always less stable than the original atom. Atoms with a partially stable outer energy level can lose, gain, or share electrons to obtain a stable outer energy level. ...
Reaction types summary
... Only the hydrogen ions from the acid combine with the hydroxide ions from the base. All of the other ions start and end the reaction in the same ionic form. They are called spectator ions (they “watch” the other ions reacting) ...
... Only the hydrogen ions from the acid combine with the hydroxide ions from the base. All of the other ions start and end the reaction in the same ionic form. They are called spectator ions (they “watch” the other ions reacting) ...
4.4 Oxidation Reduction Redox An introduction to
... Only the hydrogen ions from the acid combine with the hydroxide ions from the base. All of the other ions start and end the reaction in the same ionic form. They are called spectator ions (they “watch” the other ions reacting) ...
... Only the hydrogen ions from the acid combine with the hydroxide ions from the base. All of the other ions start and end the reaction in the same ionic form. They are called spectator ions (they “watch” the other ions reacting) ...
Chapter 2 Notes
... 1. molecules- 2 or more atoms combined; atoms share electrons in the outermost portion of their electron clouds; example: (H2O) 2. compounds- when 2 or more substances combine chemically; has properties different from the properties of each of the elements in it; example: water (H2O) 3. chemical pro ...
... 1. molecules- 2 or more atoms combined; atoms share electrons in the outermost portion of their electron clouds; example: (H2O) 2. compounds- when 2 or more substances combine chemically; has properties different from the properties of each of the elements in it; example: water (H2O) 3. chemical pro ...
Chapter 2: The Chemical Context of Life
... Molecules with nonpolar covalent bonds may have positively and negatively charged ions. Electrons are not always symmetrically distributed in such a molecule and may accumulate by chance in one part of the molecule, resulting in regions of positive and negative charges. These ever-changing regions o ...
... Molecules with nonpolar covalent bonds may have positively and negatively charged ions. Electrons are not always symmetrically distributed in such a molecule and may accumulate by chance in one part of the molecule, resulting in regions of positive and negative charges. These ever-changing regions o ...
Chapter 2
... ❹Atoms combine in simple, fixed, whole-number ratios to form compounds. What evidence is there for or against each of the postulates? What modifications are necessary to make this consistent with modern atomic theory? What is a chemical reaction? A chemical reaction is a way of rearranging atoms int ...
... ❹Atoms combine in simple, fixed, whole-number ratios to form compounds. What evidence is there for or against each of the postulates? What modifications are necessary to make this consistent with modern atomic theory? What is a chemical reaction? A chemical reaction is a way of rearranging atoms int ...
Nature of Atoms Atomic Structure
... • Element – Any substance that cannot be broken down to any other substance by ordinary chemical means ...
... • Element – Any substance that cannot be broken down to any other substance by ordinary chemical means ...
Chapter 19: Molecules and Compounds
... Ratio of atoms bonded together in a compound, i.e. X:Y General Form: AxBy where x and y are called subscripts. ...
... Ratio of atoms bonded together in a compound, i.e. X:Y General Form: AxBy where x and y are called subscripts. ...
File
... 11. What are the rows in the periodic table called and what do they show? periods 12. Using the periodic table, find the element that is found in group 2 period 3. ...
... 11. What are the rows in the periodic table called and what do they show? periods 12. Using the periodic table, find the element that is found in group 2 period 3. ...
Honors Chemistry Semester 1 Exam Review
... 11. Elements in the same group have similar ______________. They behave similarly because they have the same number of ________________. 12. Metals are located on the ____________ side of the periodic table and nonmetals are on the ____________ side. Unit 5: Bonding 1. How does a intermolecular forc ...
... 11. Elements in the same group have similar ______________. They behave similarly because they have the same number of ________________. 12. Metals are located on the ____________ side of the periodic table and nonmetals are on the ____________ side. Unit 5: Bonding 1. How does a intermolecular forc ...
AP Chapter Five Outline
... Oxidation numbers compare the charge of an uncombined atom with its actual charge in a compound. All neutral atoms have an equal number of protons and electrons and thus have no net charge. Oxidation numbers of atoms in molecular compound are assigned as though electrons were completely transfer ...
... Oxidation numbers compare the charge of an uncombined atom with its actual charge in a compound. All neutral atoms have an equal number of protons and electrons and thus have no net charge. Oxidation numbers of atoms in molecular compound are assigned as though electrons were completely transfer ...