Quantum Mechanics: Particles in Potentials
... This is the infinite set of eigenfunctions of the total energy operator,i.e. Hamiltonian, for the potential energy function corresponding to infinite, impenetrable walls at the edges of a one-dimensional box. IIb1. Normalizing the Wavefunction: The particle has to be there There is now only the cons ...
... This is the infinite set of eigenfunctions of the total energy operator,i.e. Hamiltonian, for the potential energy function corresponding to infinite, impenetrable walls at the edges of a one-dimensional box. IIb1. Normalizing the Wavefunction: The particle has to be there There is now only the cons ...
midterm answers
... kinetic energy, so U > E implies negative kinetic energy which does not exist classically, in quantum mechanics this is not a problem at all, the wave function above is for such a scenario U > E, the result of the square root is, therefore, positive, we have to set D = 0 for this scenario and end up ...
... kinetic energy, so U > E implies negative kinetic energy which does not exist classically, in quantum mechanics this is not a problem at all, the wave function above is for such a scenario U > E, the result of the square root is, therefore, positive, we have to set D = 0 for this scenario and end up ...
Mod6QM1
... * write down boundary conditions (BC) * think about what form of y(x) will fit the potential * find the wavenumbers kn=2 / * find the allowed energies En * sub k into y(x) and normalize to find the amplitude A * Now you know everything about a QM system in this potential, and you can calculate for ...
... * write down boundary conditions (BC) * think about what form of y(x) will fit the potential * find the wavenumbers kn=2 / * find the allowed energies En * sub k into y(x) and normalize to find the amplitude A * Now you know everything about a QM system in this potential, and you can calculate for ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 3. What are spherical harmonics? Are they mutually orthogonal? 4. Prove that the square of the angular momentum commutes with its z-component. 5. If A and B are two operators, then show that [A-1[A,B]] = 2B 6. What are unitary transformations? 7. Show that commuting operators have simultaneous eigen ...
... 3. What are spherical harmonics? Are they mutually orthogonal? 4. Prove that the square of the angular momentum commutes with its z-component. 5. If A and B are two operators, then show that [A-1[A,B]] = 2B 6. What are unitary transformations? 7. Show that commuting operators have simultaneous eigen ...
HOMEWORK ASSIGNMENT 5: Solutions
... many distinct energy levels make up the fine-structure of the (3p)2 state? The allowed j values are j = 0, 1, 2, so there would be 3 fine-structure levels. (f) Which j levels would shift if a contact interaction between the two valence electrons were added to the Hamiltonian? Only states with even o ...
... many distinct energy levels make up the fine-structure of the (3p)2 state? The allowed j values are j = 0, 1, 2, so there would be 3 fine-structure levels. (f) Which j levels would shift if a contact interaction between the two valence electrons were added to the Hamiltonian? Only states with even o ...
stringtheory1s
... tried to understand them in terms of oldfashioned ideas … But at a certain point the old-fashioned ideas would begin to fail, so a warning was developed that said, in effect, ‘Your old-fashioned ideas are no damn good ...
... tried to understand them in terms of oldfashioned ideas … But at a certain point the old-fashioned ideas would begin to fail, so a warning was developed that said, in effect, ‘Your old-fashioned ideas are no damn good ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
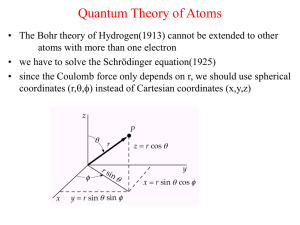
... For the wave function Ψ(φ) = Ae , where m is an integer, for 0≤φ≤2π. Determine A so that the wave function is normalized. Give the Laplacian operator in spherical polar coordinates. What are their limits of integration? CO absorbs energy in the microwave region of the spectrum at 1.93 x 1012 Hz. Thi ...
... For the wave function Ψ(φ) = Ae , where m is an integer, for 0≤φ≤2π. Determine A so that the wave function is normalized. Give the Laplacian operator in spherical polar coordinates. What are their limits of integration? CO absorbs energy in the microwave region of the spectrum at 1.93 x 1012 Hz. Thi ...
ppt - HEP Educational Outreach
... •Solution to Schrodinger Equation for Hydrogen also explains emission spectrum. •Photons are emitted when the electron in an atom drops from a higher energy level to a lower one. ...
... •Solution to Schrodinger Equation for Hydrogen also explains emission spectrum. •Photons are emitted when the electron in an atom drops from a higher energy level to a lower one. ...
wlq10
... • Messenger series of lectures, Cornell University, 1964 • Lecture 6: ‘Probability and Uncertainty – the quantum mechanical view of nature’ • The Character of Physical Law - Penguin • see the later series of Douglas Robb memorial lectures (1979) online ...
... • Messenger series of lectures, Cornell University, 1964 • Lecture 6: ‘Probability and Uncertainty – the quantum mechanical view of nature’ • The Character of Physical Law - Penguin • see the later series of Douglas Robb memorial lectures (1979) online ...
DOC - 嘉義大學
... (a) What intensity is actually available for the photoelectric effect? (b) Assuming that one photon can generate one electron, how many electrons will be emitted per second in an effective wavelength of 250 nm of the UV region. (c) Calculate the current in the phototube in units of nano-amperes (nA) ...
... (a) What intensity is actually available for the photoelectric effect? (b) Assuming that one photon can generate one electron, how many electrons will be emitted per second in an effective wavelength of 250 nm of the UV region. (c) Calculate the current in the phototube in units of nano-amperes (nA) ...
Quantum Mechanics
... • If particles can be described by waves, they are constantly moving • Location can be described by a wave function – Amplitude of wave given by Y(x,t) – Probability of being at a certain place is given by Y2(x,t) ...
... • If particles can be described by waves, they are constantly moving • Location can be described by a wave function – Amplitude of wave given by Y(x,t) – Probability of being at a certain place is given by Y2(x,t) ...
Particle in a box

In quantum mechanics, the particle in a box model (also known as the infinite potential well or the infinite square well) describes a particle free to move in a small space surrounded by impenetrable barriers. The model is mainly used as a hypothetical example to illustrate the differences between classical and quantum systems. In classical systems, for example a ball trapped inside a large box, the particle can move at any speed within the box and it is no more likely to be found at one position than another. However, when the well becomes very narrow (on the scale of a few nanometers), quantum effects become important. The particle may only occupy certain positive energy levels. Likewise, it can never have zero energy, meaning that the particle can never ""sit still"". Additionally, it is more likely to be found at certain positions than at others, depending on its energy level. The particle may never be detected at certain positions, known as spatial nodes.The particle in a box model provides one of the very few problems in quantum mechanics which can be solved analytically, without approximations. This means that the observable properties of the particle (such as its energy and position) are related to the mass of the particle and the width of the well by simple mathematical expressions. Due to its simplicity, the model allows insight into quantum effects without the need for complicated mathematics. It is one of the first quantum mechanics problems taught in undergraduate physics courses, and it is commonly used as an approximation for more complicated quantum systems.