LOYOLA COLLEGE (AUTONOMOUS), CHENNAI-600034 M.Sc. Part-A NOVEMBER 2015
... Show that the eigenvalues of Hermitian operators are real. Obtain the ground state atomic term symbol for carbon. State variation principle. Mention its significance. Mention any two point groups that obey mutual exclusion principle. Why is a molecular plane always identified to form a class by itse ...
... Show that the eigenvalues of Hermitian operators are real. Obtain the ground state atomic term symbol for carbon. State variation principle. Mention its significance. Mention any two point groups that obey mutual exclusion principle. Why is a molecular plane always identified to form a class by itse ...
Review Sheet
... Kinetic vs. potential energy Enthalpy (H) Measuring heat (q) Specific heat capacity Endothermic vs. exothermic Stoichiometry using energy (using enthalpy of reaction and balanced chemical reactions) Calculating H using Hess’s Law, Enthalpy Diagrams, and/or H° of formations Calorimetry calculations ...
... Kinetic vs. potential energy Enthalpy (H) Measuring heat (q) Specific heat capacity Endothermic vs. exothermic Stoichiometry using energy (using enthalpy of reaction and balanced chemical reactions) Calculating H using Hess’s Law, Enthalpy Diagrams, and/or H° of formations Calorimetry calculations ...
SCHRÖDINGER EQUATION FOR A PARTICLE ON A CURVED SPACE AND SUPERINTEGRABILITY
... space of common eigenstates. Three different orthogonal coordinate systems are determined, one for each case. It is shown how the Schrödinger equation can be rendered separable in each of the cases. ...
... space of common eigenstates. Three different orthogonal coordinate systems are determined, one for each case. It is shown how the Schrödinger equation can be rendered separable in each of the cases. ...
Modern Physics Lesson 3
... Conclusion: A photon is a particle of light that has energy and momentum. However, photons have no mass and travel at the speed of light, c. deBroglie Wavelength Louis deBroglie proposed in 1923 that if waves behave like particles, then particles should also behave like waves! This was the beginnin ...
... Conclusion: A photon is a particle of light that has energy and momentum. However, photons have no mass and travel at the speed of light, c. deBroglie Wavelength Louis deBroglie proposed in 1923 that if waves behave like particles, then particles should also behave like waves! This was the beginnin ...
Transparancies for Revision Lecture - University of Manchester
... Energy splitting depends on l even in absence of magnetic field. ...
... Energy splitting depends on l even in absence of magnetic field. ...
Exercises in Statistical Mechanics
... Based on course by Doron Cohen, has to be proofed Department of Physics, Ben-Gurion University, Beer-Sheva 84105, Israel This exercises pool is intended for a graduate course in “statistical mechanics”. Some of the problems are original, while other were assembled from various undocumented sources. ...
... Based on course by Doron Cohen, has to be proofed Department of Physics, Ben-Gurion University, Beer-Sheva 84105, Israel This exercises pool is intended for a graduate course in “statistical mechanics”. Some of the problems are original, while other were assembled from various undocumented sources. ...
BasicQuantumMechanics18And20January2017
... • It is impossible to simultaneously describe with absolute accuracy the energy of a particle and the instant of time the particle has this energy ...
... • It is impossible to simultaneously describe with absolute accuracy the energy of a particle and the instant of time the particle has this energy ...
Unit 2 Intro Worksheet - Coral Gables Senior High
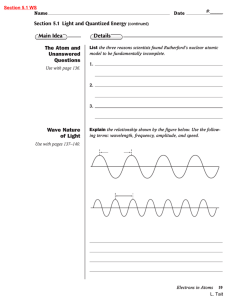
... ____ 7. discrete bundle of electromagnetic energy ____ 8. energy needed to move an electron from one energy level to another ____ 9. number of wave cycles passing a point per unit of time ____ 10. distance between wave crests ____ 11. separation of light into different wavelengths ____ 12. frequenci ...
... ____ 7. discrete bundle of electromagnetic energy ____ 8. energy needed to move an electron from one energy level to another ____ 9. number of wave cycles passing a point per unit of time ____ 10. distance between wave crests ____ 11. separation of light into different wavelengths ____ 12. frequenci ...
Key Concepts for Exam #2
... If the frequency of incident light is above the threshold frequency, then as the intensity of light increases, the kinetic energy of ejected electrons remains constant and the number of electrons increases. In addition, as the frequency of light increases, the kinetic energy of ejected electrons inc ...
... If the frequency of incident light is above the threshold frequency, then as the intensity of light increases, the kinetic energy of ejected electrons remains constant and the number of electrons increases. In addition, as the frequency of light increases, the kinetic energy of ejected electrons inc ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI M.Sc. THIRD
... 14. Solve the Schroedinger wave equation and obtain the energy eigenvalues of a particle of mass ‘m’ in a three dimensional square well potential of side ‘L’. What is the degeneracy ...
... 14. Solve the Schroedinger wave equation and obtain the energy eigenvalues of a particle of mass ‘m’ in a three dimensional square well potential of side ‘L’. What is the degeneracy ...
Group and phase velocity
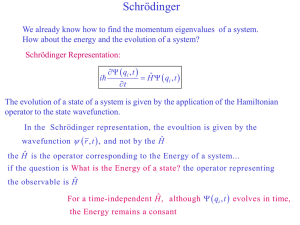
... The evolution of a state of a system is given by the application of the Hamiltonian operator to the state wavefunction. In the Schrodinger representation, the evoultion is given by the wavefunction r , t , and not by the Hˆ ...
... The evolution of a state of a system is given by the application of the Hamiltonian operator to the state wavefunction. In the Schrodinger representation, the evoultion is given by the wavefunction r , t , and not by the Hˆ ...
Particle in a box

In quantum mechanics, the particle in a box model (also known as the infinite potential well or the infinite square well) describes a particle free to move in a small space surrounded by impenetrable barriers. The model is mainly used as a hypothetical example to illustrate the differences between classical and quantum systems. In classical systems, for example a ball trapped inside a large box, the particle can move at any speed within the box and it is no more likely to be found at one position than another. However, when the well becomes very narrow (on the scale of a few nanometers), quantum effects become important. The particle may only occupy certain positive energy levels. Likewise, it can never have zero energy, meaning that the particle can never ""sit still"". Additionally, it is more likely to be found at certain positions than at others, depending on its energy level. The particle may never be detected at certain positions, known as spatial nodes.The particle in a box model provides one of the very few problems in quantum mechanics which can be solved analytically, without approximations. This means that the observable properties of the particle (such as its energy and position) are related to the mass of the particle and the width of the well by simple mathematical expressions. Due to its simplicity, the model allows insight into quantum effects without the need for complicated mathematics. It is one of the first quantum mechanics problems taught in undergraduate physics courses, and it is commonly used as an approximation for more complicated quantum systems.