CHM 50- Class activity
... The retina of a human eye can detect light when radiant energy incident on it is at least 4.0 x 10-17 J. For light of 5.85 nm wavelength , how many photons does this energy correspond to? ...
... The retina of a human eye can detect light when radiant energy incident on it is at least 4.0 x 10-17 J. For light of 5.85 nm wavelength , how many photons does this energy correspond to? ...
Quantum Theory 1 - Home Exercise 4
... Find the probability current associated with this wave function, Interpret the different terms and show that if |A| = |B| the probability current vanishes. 4. Particle on a ring - Consider a particle that is free to move on a ring of circumference L, such that ψ(x, t) = ψ(x + L, t) (a) Find the norm ...
... Find the probability current associated with this wave function, Interpret the different terms and show that if |A| = |B| the probability current vanishes. 4. Particle on a ring - Consider a particle that is free to move on a ring of circumference L, such that ψ(x, t) = ψ(x + L, t) (a) Find the norm ...
4.8-Quantum Mechanics
... Dirac’s theory removed the paradox of particle-wave duality: It showed that if a particle was probed in a way that was meant to demonstrate its particle like properties - it would appear to be a particle…... …….if it was probed in a way that was meant to demonstrate its wave like properties - it wou ...
... Dirac’s theory removed the paradox of particle-wave duality: It showed that if a particle was probed in a way that was meant to demonstrate its particle like properties - it would appear to be a particle…... …….if it was probed in a way that was meant to demonstrate its wave like properties - it wou ...
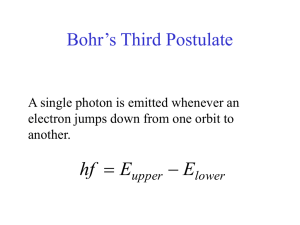
Bohr´s Third Postulate
... waves? Could this experiment distinguish between neutral particles and an e/m wave? 3. If a metal surface is illuminated by light at a single frequency, why don’t all the photoelectrons have the same kinetic energy when they leave the metal’s surface? 4. What property of the emitted electrons depend ...
... waves? Could this experiment distinguish between neutral particles and an e/m wave? 3. If a metal surface is illuminated by light at a single frequency, why don’t all the photoelectrons have the same kinetic energy when they leave the metal’s surface? 4. What property of the emitted electrons depend ...
1. Crystal Properties and Growth of Semiconductors
... radiation emanating from them Bohr postulates: 1) Electron exists in certain stable circular orbits about the nucleus and does not give off radiation 2) Electron may shift to an orbit of higher or lower energy by absorbing or emitting a photon of energy hf 3) Angular momentum is quantized p =m v r = ...
... radiation emanating from them Bohr postulates: 1) Electron exists in certain stable circular orbits about the nucleus and does not give off radiation 2) Electron may shift to an orbit of higher or lower energy by absorbing or emitting a photon of energy hf 3) Angular momentum is quantized p =m v r = ...
Document
... (x,t ) contains within it all the information that can be known about the particle (x, t ) is an infinite set of numbers corresponding to the wavefunction value at every point x at time t ...
... (x,t ) contains within it all the information that can be known about the particle (x, t ) is an infinite set of numbers corresponding to the wavefunction value at every point x at time t ...
Powerpoint handout
... proposing that electrons in atoms could have only certain energies, and that light was given off when an electron underwent a transition from a higher energy level to a lower one. ...
... proposing that electrons in atoms could have only certain energies, and that light was given off when an electron underwent a transition from a higher energy level to a lower one. ...
Lecture 24: Quantum mechanics
... century, Raleigh showed that the energy density per unit wavelength should follow: ...
... century, Raleigh showed that the energy density per unit wavelength should follow: ...
Ideas of Modern Physics
... 2. A beta particle, gamma ray, and alpha particle all have the same momentum. Which has the longest wavelength? a. beta particle. b. gamma ray. c. alpha particle. d. all the same. e. depends on gamma ray energy. 3. Particular red (600 nm) and blue (300 nm) lasers both produce 10 mW of power. How do ...
... 2. A beta particle, gamma ray, and alpha particle all have the same momentum. Which has the longest wavelength? a. beta particle. b. gamma ray. c. alpha particle. d. all the same. e. depends on gamma ray energy. 3. Particular red (600 nm) and blue (300 nm) lasers both produce 10 mW of power. How do ...
Particle in a box

In quantum mechanics, the particle in a box model (also known as the infinite potential well or the infinite square well) describes a particle free to move in a small space surrounded by impenetrable barriers. The model is mainly used as a hypothetical example to illustrate the differences between classical and quantum systems. In classical systems, for example a ball trapped inside a large box, the particle can move at any speed within the box and it is no more likely to be found at one position than another. However, when the well becomes very narrow (on the scale of a few nanometers), quantum effects become important. The particle may only occupy certain positive energy levels. Likewise, it can never have zero energy, meaning that the particle can never ""sit still"". Additionally, it is more likely to be found at certain positions than at others, depending on its energy level. The particle may never be detected at certain positions, known as spatial nodes.The particle in a box model provides one of the very few problems in quantum mechanics which can be solved analytically, without approximations. This means that the observable properties of the particle (such as its energy and position) are related to the mass of the particle and the width of the well by simple mathematical expressions. Due to its simplicity, the model allows insight into quantum effects without the need for complicated mathematics. It is one of the first quantum mechanics problems taught in undergraduate physics courses, and it is commonly used as an approximation for more complicated quantum systems.