Lecture 18: Intro. to Quantum Mechanics
... • Louis de Broglie suggests that for the e- orbits envisioned by Bohr, only certain orbits are allowed since they satisfy the standing wave condition. ...
... • Louis de Broglie suggests that for the e- orbits envisioned by Bohr, only certain orbits are allowed since they satisfy the standing wave condition. ...
PHYSICS 215 - Thermodynamics and Modern Physics Name:
... What is the minimum angle between L and the z axis? ...
... What is the minimum angle between L and the z axis? ...
3.4oquantum.4u
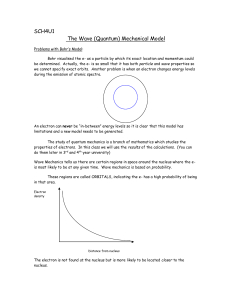
... we cannot specify exact orbits. Another problem is when an electron changes energy levels during the emission of atomic spectra. ...
... we cannot specify exact orbits. Another problem is when an electron changes energy levels during the emission of atomic spectra. ...
Generalized Momentum Operators
... interval (confined to the interior of a box): x ∈ [a, b]. For while we can expect to have ψ(a) = ψ(b) = 0, we cannot expect to have ψ (n) (a) = ψ (n) (b). Look, for example, to the energy eigenfunctions ...
... interval (confined to the interior of a box): x ∈ [a, b]. For while we can expect to have ψ(a) = ψ(b) = 0, we cannot expect to have ψ (n) (a) = ψ (n) (b). Look, for example, to the energy eigenfunctions ...
Class23
... Quantum mechanics challenges our physical intuition but it is the way things really work. Particles are described with a wave function Y(x,t) which describes the propagation through space and time (when unobserved). ...
... Quantum mechanics challenges our physical intuition but it is the way things really work. Particles are described with a wave function Y(x,t) which describes the propagation through space and time (when unobserved). ...
lect10
... accordance with Newton’s Laws but do not radiate energy an atom emits or absorbs energy only when an electron moves from one stable state to another ...
... accordance with Newton’s Laws but do not radiate energy an atom emits or absorbs energy only when an electron moves from one stable state to another ...
Task 1
... "ground state energy" or zero-point energy. In the __________ state, according to quantum mechanics, an ___________ performs null oscillations and its average kinetic energy is positive. It is not obvious that this is significant, because normally the zero of energy is not a physically meaningful qu ...
... "ground state energy" or zero-point energy. In the __________ state, according to quantum mechanics, an ___________ performs null oscillations and its average kinetic energy is positive. It is not obvious that this is significant, because normally the zero of energy is not a physically meaningful qu ...
Lecture #3
... A moving “particle” is described by a superposition of a great many sin and cos waves which constructively interfere to give a Gaussian probability near a certain point but destructively interfere everywhere else. The Gaussian “wave packet” moves according to the kinetic energy given by the average ...
... A moving “particle” is described by a superposition of a great many sin and cos waves which constructively interfere to give a Gaussian probability near a certain point but destructively interfere everywhere else. The Gaussian “wave packet” moves according to the kinetic energy given by the average ...
CH-103 Tutorial-1
... given by − h d ψ2 (θ ) = Enψ (θ ) , where I=ma2 is the moment of inertia and is the angle that 2I d θ describes the position of the particle on the circular ring. Suggest acceptable solution, permissible values of the quantum number n and obtain the expression for the eigenvalue En using appropria ...
... given by − h d ψ2 (θ ) = Enψ (θ ) , where I=ma2 is the moment of inertia and is the angle that 2I d θ describes the position of the particle on the circular ring. Suggest acceptable solution, permissible values of the quantum number n and obtain the expression for the eigenvalue En using appropria ...
Quantum Problems 1. Consider a quantum system whose state at
... 1. Consider a quantum system whose state at time t1 is given by |Ψ(t1 )i = √12 (|ψ1 i + |ψ2 i), where |ψ1 i and |ψ2 i are energy eigenstates with eigenvalues E1 and E2 respectively. (E1 6= E2 .) (a) Calculate the uncertainty ∆E of the system, as well as the first time t2 > t1 at which |Ψ(t2 )i becom ...
... 1. Consider a quantum system whose state at time t1 is given by |Ψ(t1 )i = √12 (|ψ1 i + |ψ2 i), where |ψ1 i and |ψ2 i are energy eigenstates with eigenvalues E1 and E2 respectively. (E1 6= E2 .) (a) Calculate the uncertainty ∆E of the system, as well as the first time t2 > t1 at which |Ψ(t2 )i becom ...
tutorial questions on special relativity
... 9. A particle in an infinite well is in the ground state with an energy of 1.26 eV. How much energy must be added to the particle to reach the second excited state (n = 3)? The third excited state (n = 4)? (Krane, P4, pg. 170) 10. An electron is trapped in a one-dimensional well of width 0.132 nm. T ...
... 9. A particle in an infinite well is in the ground state with an energy of 1.26 eV. How much energy must be added to the particle to reach the second excited state (n = 3)? The third excited state (n = 4)? (Krane, P4, pg. 170) 10. An electron is trapped in a one-dimensional well of width 0.132 nm. T ...
Particle in a box

In quantum mechanics, the particle in a box model (also known as the infinite potential well or the infinite square well) describes a particle free to move in a small space surrounded by impenetrable barriers. The model is mainly used as a hypothetical example to illustrate the differences between classical and quantum systems. In classical systems, for example a ball trapped inside a large box, the particle can move at any speed within the box and it is no more likely to be found at one position than another. However, when the well becomes very narrow (on the scale of a few nanometers), quantum effects become important. The particle may only occupy certain positive energy levels. Likewise, it can never have zero energy, meaning that the particle can never ""sit still"". Additionally, it is more likely to be found at certain positions than at others, depending on its energy level. The particle may never be detected at certain positions, known as spatial nodes.The particle in a box model provides one of the very few problems in quantum mechanics which can be solved analytically, without approximations. This means that the observable properties of the particle (such as its energy and position) are related to the mass of the particle and the width of the well by simple mathematical expressions. Due to its simplicity, the model allows insight into quantum effects without the need for complicated mathematics. It is one of the first quantum mechanics problems taught in undergraduate physics courses, and it is commonly used as an approximation for more complicated quantum systems.