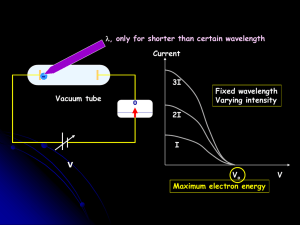
REVIEW OF WAVE MECHANICS
... Because these commutation relations are all nonzero they represent incompatible observables. This means that two different components of angular momentum cannot be measured simultaneously without uncertainties, since they do not have the same eigenfunctions. ...
... Because these commutation relations are all nonzero they represent incompatible observables. This means that two different components of angular momentum cannot be measured simultaneously without uncertainties, since they do not have the same eigenfunctions. ...
Physics and the Quantum Mechanical Model
... Only way to know precise position of a subatomic particle is for a photon (light) to collide with it. The collision will change the velocity of the particle Therefore, we can’t know both at the exact same time. ...
... Only way to know precise position of a subatomic particle is for a photon (light) to collide with it. The collision will change the velocity of the particle Therefore, we can’t know both at the exact same time. ...
particle in a box the uncertainty principle
... A free particle, outside the box, can have any energy, and any wavelength. When you put the particle in the box, only certain wavelengths and energies are allowed (and note that zero is not one of the allowed energies). You most likely will have to add or remove energy to put a free particle into a ...
... A free particle, outside the box, can have any energy, and any wavelength. When you put the particle in the box, only certain wavelengths and energies are allowed (and note that zero is not one of the allowed energies). You most likely will have to add or remove energy to put a free particle into a ...
Document
... from Niels Bohr who explained experimentally observed discrete nature of atomic spectrum of Hydrogen. In spite of its immediate success in providing theoretical account of the spectrum and other nature of Hydrogen atom, a complete understanding of Bohr’s atom came only after de Broglie’s conjecture ...
... from Niels Bohr who explained experimentally observed discrete nature of atomic spectrum of Hydrogen. In spite of its immediate success in providing theoretical account of the spectrum and other nature of Hydrogen atom, a complete understanding of Bohr’s atom came only after de Broglie’s conjecture ...
Physics 880.06: Problem Set 7
... Tc (B) for (i) B parallel to the z axis; (ii) B parallel to the x axis. Assume that the field B is specified. Your answers should involve the effective masses mab and mc . (b). (10 pts.) Assuming that the order parameter ψ is independent of position, obtain an expression for the current density J i ...
... Tc (B) for (i) B parallel to the z axis; (ii) B parallel to the x axis. Assume that the field B is specified. Your answers should involve the effective masses mab and mc . (b). (10 pts.) Assuming that the order parameter ψ is independent of position, obtain an expression for the current density J i ...
notes on Bohr and the hydrogen spectrum
... which is in the infrared. But the important thing is that each pair of integers gives an energy. n1 and n2 are whole numbers, so the spectrum has certain discrete values, rather than a continuum. By the way, when the electron 'jumps' from one state (n2) to another (n1), this was called a quantum jum ...
... which is in the infrared. But the important thing is that each pair of integers gives an energy. n1 and n2 are whole numbers, so the spectrum has certain discrete values, rather than a continuum. By the way, when the electron 'jumps' from one state (n2) to another (n1), this was called a quantum jum ...
Quantum Mechanics
... Treating the orbit of an electron as a continuous wave, the path length (2πr) must be equal to a whole number of wavelengths ...
... Treating the orbit of an electron as a continuous wave, the path length (2πr) must be equal to a whole number of wavelengths ...
Particle in a box

In quantum mechanics, the particle in a box model (also known as the infinite potential well or the infinite square well) describes a particle free to move in a small space surrounded by impenetrable barriers. The model is mainly used as a hypothetical example to illustrate the differences between classical and quantum systems. In classical systems, for example a ball trapped inside a large box, the particle can move at any speed within the box and it is no more likely to be found at one position than another. However, when the well becomes very narrow (on the scale of a few nanometers), quantum effects become important. The particle may only occupy certain positive energy levels. Likewise, it can never have zero energy, meaning that the particle can never ""sit still"". Additionally, it is more likely to be found at certain positions than at others, depending on its energy level. The particle may never be detected at certain positions, known as spatial nodes.The particle in a box model provides one of the very few problems in quantum mechanics which can be solved analytically, without approximations. This means that the observable properties of the particle (such as its energy and position) are related to the mass of the particle and the width of the well by simple mathematical expressions. Due to its simplicity, the model allows insight into quantum effects without the need for complicated mathematics. It is one of the first quantum mechanics problems taught in undergraduate physics courses, and it is commonly used as an approximation for more complicated quantum systems.