Ch. 40
... 2 to the n = 1 level. a photon with A = 122 nm is emitted. (a) If the atom is modeled as an electron in a one-dimensional box, what is the width of the box in order fOl' the 11 - 2 to n - 1 transition to correspond to emission of a photon of this enetgy? (b) For a box with the width calculated in pa ...
... 2 to the n = 1 level. a photon with A = 122 nm is emitted. (a) If the atom is modeled as an electron in a one-dimensional box, what is the width of the box in order fOl' the 11 - 2 to n - 1 transition to correspond to emission of a photon of this enetgy? (b) For a box with the width calculated in pa ...
collapses - Marc Madou
... In the late 18th century the mathematician Pierre Simon de Laplace (17491827) encapsulated classical determinism as follows: “…if at one time we knew the positions and motion of all the particles in the Universe, then we could calculate their behavior at any other time, in the past or the future.” ...
... In the late 18th century the mathematician Pierre Simon de Laplace (17491827) encapsulated classical determinism as follows: “…if at one time we knew the positions and motion of all the particles in the Universe, then we could calculate their behavior at any other time, in the past or the future.” ...
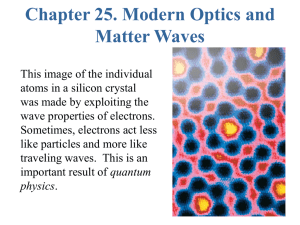
Chapt25_VGO
... Problems: This model predicts a continuous spectrum. If you calculate the rate at which energy is radiated, all electrons would quickly spiral into the nucleus. Neither is observed. ...
... Problems: This model predicts a continuous spectrum. If you calculate the rate at which energy is radiated, all electrons would quickly spiral into the nucleus. Neither is observed. ...
5.11 Harmonic Oscillator
... Before we continue, let’s think about harmonic oscillators… Classically, all energies are allowed. What will QM say? Only quantized energies? Classically, an energy of zero is allowed. What will QM say? Nonzero, like particle in box? Classically, the oscillator can't exist in a state in "forbidden" ...
... Before we continue, let’s think about harmonic oscillators… Classically, all energies are allowed. What will QM say? Only quantized energies? Classically, an energy of zero is allowed. What will QM say? Nonzero, like particle in box? Classically, the oscillator can't exist in a state in "forbidden" ...
Proof that the de Broglie-Einstein velocity equation is valid for the
... [3, 4]. His ideas were supported by the electron diffraction experiments of Davisson and Germer [5]. One of the most important inventions of de Broglie was the derivation of the de Broglie-Einstein velocity equation [6]. This equation gives the relation between the phase and group velocities of a ma ...
... [3, 4]. His ideas were supported by the electron diffraction experiments of Davisson and Germer [5]. One of the most important inventions of de Broglie was the derivation of the de Broglie-Einstein velocity equation [6]. This equation gives the relation between the phase and group velocities of a ma ...
Quantum Physics
... metastable state – state where stimulated emission lifetime is longer than spontaneous emission lifetime, state where stimulated emission is more likely than spontaneous emission population inversion – when there are more atoms in state 2 than in state 1, a necessary condition for continued lasing s ...
... metastable state – state where stimulated emission lifetime is longer than spontaneous emission lifetime, state where stimulated emission is more likely than spontaneous emission population inversion – when there are more atoms in state 2 than in state 1, a necessary condition for continued lasing s ...
Lecture notes, part 2
... = 0. If all these states are also normalized, they are said to be “orthonormal”. Case 2: degenerate. En can equal Em even if n 6= m. The proof is more complicated than the non-degenerate case. We need to use the GramSchmitt orthogonalization procedure to construct orthogonal states by taking appropr ...
... = 0. If all these states are also normalized, they are said to be “orthonormal”. Case 2: degenerate. En can equal Em even if n 6= m. The proof is more complicated than the non-degenerate case. We need to use the GramSchmitt orthogonalization procedure to construct orthogonal states by taking appropr ...
Chapter 7: The Quantum Mechanical Model of the Atom I. The
... 1. Bohr s major idea was that the energy states of the atom were _________, and that the amount of energy in the atom was related to the electron s position in the atom. 2. The electrons travel in orbits that are at a fixed distance from the nucleus. ...
... 1. Bohr s major idea was that the energy states of the atom were _________, and that the amount of energy in the atom was related to the electron s position in the atom. 2. The electrons travel in orbits that are at a fixed distance from the nucleus. ...
Chapter 4
... Hund’s Rule – each orbital is filled with 1efirst and then the 2nd e- will fill Pauli Exclusion Principle – no 2 e- in the same atom can have the same set of QN ...
... Hund’s Rule – each orbital is filled with 1efirst and then the 2nd e- will fill Pauli Exclusion Principle – no 2 e- in the same atom can have the same set of QN ...
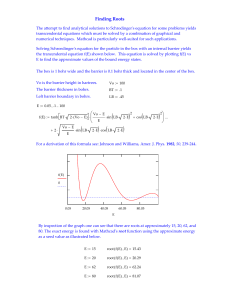
Supplement 13A
... In our discussion of angular momentum in Chapter 8 we found that the assumption of invariance of the Hamiltonian under rotations led to the appearance of a new constant of motion, the angular momentum. In this supplement we show that the assumption of invariance under spatial displacement leads to t ...
... In our discussion of angular momentum in Chapter 8 we found that the assumption of invariance of the Hamiltonian under rotations led to the appearance of a new constant of motion, the angular momentum. In this supplement we show that the assumption of invariance under spatial displacement leads to t ...
Probing contextuality with superconducting quantum circuits Talk 27. Oct. 2015 ABSTRACT:
... Talk 27. Oct. 2015 ...
... Talk 27. Oct. 2015 ...
Energy transfer of a chaotic particle in a classical oscillating
... The problem of dissipation, friction and energy transfer between classical or quantum systems has traditionally been studied phenomenologically. Recently, a more basic approach to these problems has appeared based on classical or quantum chaotic ideas [1,2]. In this paper we consider the energy tran ...
... The problem of dissipation, friction and energy transfer between classical or quantum systems has traditionally been studied phenomenologically. Recently, a more basic approach to these problems has appeared based on classical or quantum chaotic ideas [1,2]. In this paper we consider the energy tran ...
Particle in a box

In quantum mechanics, the particle in a box model (also known as the infinite potential well or the infinite square well) describes a particle free to move in a small space surrounded by impenetrable barriers. The model is mainly used as a hypothetical example to illustrate the differences between classical and quantum systems. In classical systems, for example a ball trapped inside a large box, the particle can move at any speed within the box and it is no more likely to be found at one position than another. However, when the well becomes very narrow (on the scale of a few nanometers), quantum effects become important. The particle may only occupy certain positive energy levels. Likewise, it can never have zero energy, meaning that the particle can never ""sit still"". Additionally, it is more likely to be found at certain positions than at others, depending on its energy level. The particle may never be detected at certain positions, known as spatial nodes.The particle in a box model provides one of the very few problems in quantum mechanics which can be solved analytically, without approximations. This means that the observable properties of the particle (such as its energy and position) are related to the mass of the particle and the width of the well by simple mathematical expressions. Due to its simplicity, the model allows insight into quantum effects without the need for complicated mathematics. It is one of the first quantum mechanics problems taught in undergraduate physics courses, and it is commonly used as an approximation for more complicated quantum systems.