Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical
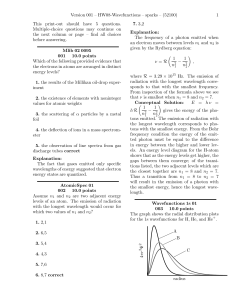
... The Balmer series occurs in the visible and near ultraviolet regions. There are lines in the hydrogen atomic spectrum in other regions; in fact there are series of lines similar to the Balmer series in the ultraviolet and in the infrared region. ...
... The Balmer series occurs in the visible and near ultraviolet regions. There are lines in the hydrogen atomic spectrum in other regions; in fact there are series of lines similar to the Balmer series in the ultraviolet and in the infrared region. ...
Postulate 1
... eigenvalues, eigenfunctions and eigenvalue equations is needed before moving to, for example, the postulates of quantum mechanics. Some of this material may be familiar from mathematics courses. The “eigenfunctions” that are useful in describing particles with wave properties are of familiar form (a ...
... eigenvalues, eigenfunctions and eigenvalue equations is needed before moving to, for example, the postulates of quantum mechanics. Some of this material may be familiar from mathematics courses. The “eigenfunctions” that are useful in describing particles with wave properties are of familiar form (a ...
Copenhagen Interpretation (of quantum physics)
... The key concept is the so-called ‘collapse of the wave function’, In seeking to explain how an entity such as a photon or an electron could ‘travel as a wave but arrive as a particle’, Bohr and his colleagues said it was the act of observing the wave that made it ‘collapse’ to become a particle… But ...
... The key concept is the so-called ‘collapse of the wave function’, In seeking to explain how an entity such as a photon or an electron could ‘travel as a wave but arrive as a particle’, Bohr and his colleagues said it was the act of observing the wave that made it ‘collapse’ to become a particle… But ...
量子力學
... 9. Find bound-state eigenenergies of a particle in an attractive potential, V(x)=–aVo (x). 10. A particle is moving along the x-axis. Find the transmission probability of this particle through a delta-function potential barrier at the origin V(x)= aVo (x). 11. (a) Determine eigenenergies and cor ...
... 9. Find bound-state eigenenergies of a particle in an attractive potential, V(x)=–aVo (x). 10. A particle is moving along the x-axis. Find the transmission probability of this particle through a delta-function potential barrier at the origin V(x)= aVo (x). 11. (a) Determine eigenenergies and cor ...
chem6V19_postulates
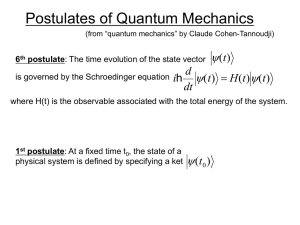
... where H(t) is the observable associated with the total energy of the system. ...
... where H(t) is the observable associated with the total energy of the system. ...
3.2 Conserved Properties/Constants of Motion
... only the phase changes as a function of time. A successive measurement will find always the same Eigenvalue. The energy and the expectation value of the operator A are thus always measurable at the same time. The state of as system is defined completely if all expectation values of those operators a ...
... only the phase changes as a function of time. A successive measurement will find always the same Eigenvalue. The energy and the expectation value of the operator A are thus always measurable at the same time. The state of as system is defined completely if all expectation values of those operators a ...
- BUGS McGill
... find the most probable distance from the nucleus of finding the electron (assuming spherical symmetry). ...
... find the most probable distance from the nucleus of finding the electron (assuming spherical symmetry). ...
Particle in a box

In quantum mechanics, the particle in a box model (also known as the infinite potential well or the infinite square well) describes a particle free to move in a small space surrounded by impenetrable barriers. The model is mainly used as a hypothetical example to illustrate the differences between classical and quantum systems. In classical systems, for example a ball trapped inside a large box, the particle can move at any speed within the box and it is no more likely to be found at one position than another. However, when the well becomes very narrow (on the scale of a few nanometers), quantum effects become important. The particle may only occupy certain positive energy levels. Likewise, it can never have zero energy, meaning that the particle can never ""sit still"". Additionally, it is more likely to be found at certain positions than at others, depending on its energy level. The particle may never be detected at certain positions, known as spatial nodes.The particle in a box model provides one of the very few problems in quantum mechanics which can be solved analytically, without approximations. This means that the observable properties of the particle (such as its energy and position) are related to the mass of the particle and the width of the well by simple mathematical expressions. Due to its simplicity, the model allows insight into quantum effects without the need for complicated mathematics. It is one of the first quantum mechanics problems taught in undergraduate physics courses, and it is commonly used as an approximation for more complicated quantum systems.