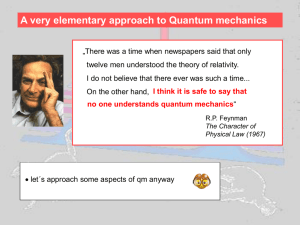
Snímek 1
... nuclei. Half-integral spin of this nucleon is summed with integral angular momentum of rest of nucleus half-integral spin of nucleus. Proton and neutron are left over at odd-odd nuclei integral spin of nucleus. Shell model: 1) All nucleons create potential, which we describe by 50 MeV deep squar ...
... nuclei. Half-integral spin of this nucleon is summed with integral angular momentum of rest of nucleus half-integral spin of nucleus. Proton and neutron are left over at odd-odd nuclei integral spin of nucleus. Shell model: 1) All nucleons create potential, which we describe by 50 MeV deep squar ...
Notes-15 - KSU Physics
... where the first integral is the classical expression for the interaction energy between two electron clouds represented by the modulus square of each wavefunction. The second term has no classical analog-- it is the exchange interaction. The latter is a consequence of quantum effect, due to that the ...
... where the first integral is the classical expression for the interaction energy between two electron clouds represented by the modulus square of each wavefunction. The second term has no classical analog-- it is the exchange interaction. The latter is a consequence of quantum effect, due to that the ...
Chapter 2 class slides
... If the frequency is constant and the Intensity is the changed, the rate at which the photoelectrons emit is changed but the MAX KE is not affected. ...
... If the frequency is constant and the Intensity is the changed, the rate at which the photoelectrons emit is changed but the MAX KE is not affected. ...
3rd Quarter Test
... a) a polar covalent bond with an electronegativity difference of zero b) a polar covalent bond with an electronegativity difference between zero and 1.7 c) a non-polar covalent bond with an electronegativity difference of zero d) a non-polar covalent bond with an electronegativity difference between ...
... a) a polar covalent bond with an electronegativity difference of zero b) a polar covalent bond with an electronegativity difference between zero and 1.7 c) a non-polar covalent bond with an electronegativity difference of zero d) a non-polar covalent bond with an electronegativity difference between ...
Energy Levels Of Hydrogen Atom Using Ladder Operators
... This time the energy is !ω more than that of the ground state. When † is applied again, the energy will increase by a further !ω, ie two units of energy above the ground state, and so on. In this context, † and  are known as the ladder operators. The figure below [1] indicates the raising and ...
... This time the energy is !ω more than that of the ground state. When † is applied again, the energy will increase by a further !ω, ie two units of energy above the ground state, and so on. In this context, † and  are known as the ladder operators. The figure below [1] indicates the raising and ...
Chapter 3 Study Guide
... i. Tells you the order in which electrons fill up an atom. b. Rules to obey when determining electron configurations: i. Pauli Exclusion Principle: no two electrons in the same orbital can have the same 4 quantum numbers. ii. aufbau principle: electrons fill up the lowest available energy levels fir ...
... i. Tells you the order in which electrons fill up an atom. b. Rules to obey when determining electron configurations: i. Pauli Exclusion Principle: no two electrons in the same orbital can have the same 4 quantum numbers. ii. aufbau principle: electrons fill up the lowest available energy levels fir ...
5.4 Quantum Devices Energy Levels in a Single Quantum Well
... Since single atoms may also be described as SQWs (for one electron you just have the hydrogen atom type with a Coulomb potential), we must expect that the wave function of the electrons start to overlap as soon as the single SQWs in the MQW structure are close enough. The situation is completely ana ...
... Since single atoms may also be described as SQWs (for one electron you just have the hydrogen atom type with a Coulomb potential), we must expect that the wave function of the electrons start to overlap as soon as the single SQWs in the MQW structure are close enough. The situation is completely ana ...
mrnotes1 - University of Warwick
... treated as point masses with energies of interaction between them. Almost all the mass is in the nucleus, essentially a point (10-15 m) compared to atomic size (10-10 m), so this defines the natural centre. The detail of interactions comes mainly from the electrons, but as long as stay bound to the ...
... treated as point masses with energies of interaction between them. Almost all the mass is in the nucleus, essentially a point (10-15 m) compared to atomic size (10-10 m), so this defines the natural centre. The detail of interactions comes mainly from the electrons, but as long as stay bound to the ...
study guide first semester chemistry
... 1. Write the balanced equation for the following: (include the state of each reactant and product) a. magnesium reacts with nitrogen to produce magnesium nitride. (3Mg(s) + N2(g) Mg3N2(s) b. silver nitrate reacts with copper to form copper(II) nitrate and silver. (2AgNO3(aq) + Cu Cu(NO3)2(aq) +2 ...
... 1. Write the balanced equation for the following: (include the state of each reactant and product) a. magnesium reacts with nitrogen to produce magnesium nitride. (3Mg(s) + N2(g) Mg3N2(s) b. silver nitrate reacts with copper to form copper(II) nitrate and silver. (2AgNO3(aq) + Cu Cu(NO3)2(aq) +2 ...
Atomic Structure 1. Historical perspective of the model of the atom a
... a.) In 1803, John Dalton proposed the atomic theory which stated that all matter is made of atoms, atoms of the same type of element have the same chemical properties, compounds are formed by two or more different types of atoms, and that a chemical reaction involves either, joining, separating, or ...
... a.) In 1803, John Dalton proposed the atomic theory which stated that all matter is made of atoms, atoms of the same type of element have the same chemical properties, compounds are formed by two or more different types of atoms, and that a chemical reaction involves either, joining, separating, or ...