Problem Set 1 - MIT OpenCourseWare
... 2. (25 points) Dimensional Analysis: Two Kinds of Quantum Gravity (a) Gravitational bound states Consider a particle sitting on a table which is kept from floating away only by the force of gravity. This system is characterized by just three physical parameters, the mass of the particle, m, the acce ...
... 2. (25 points) Dimensional Analysis: Two Kinds of Quantum Gravity (a) Gravitational bound states Consider a particle sitting on a table which is kept from floating away only by the force of gravity. This system is characterized by just three physical parameters, the mass of the particle, m, the acce ...
instroduction_a_final
... system. For example: a mathematical function of object in XY plane: y = x^2+3 or f(x)= x^2 + 3, from the function we can know the position of the object by given x value. A much better sample of a wavefunction is: . That is used to describe an electron in a hydrogen atom. Where r is the distance of ...
... system. For example: a mathematical function of object in XY plane: y = x^2+3 or f(x)= x^2 + 3, from the function we can know the position of the object by given x value. A much better sample of a wavefunction is: . That is used to describe an electron in a hydrogen atom. Where r is the distance of ...
Physical Chemistry II – Exam 1 SOLUTIONS
... 1.) (17 points) When light with a wavelength of 3000 Å shines on a metal surface, the kinetic energy of ejected electrons is 2.960×10–19 J. When light with a wavelength of 5400 Å shines on the same metal surface, the kinetic energy of ejected electrons is 1.362×10–21 J. From this information, determ ...
... 1.) (17 points) When light with a wavelength of 3000 Å shines on a metal surface, the kinetic energy of ejected electrons is 2.960×10–19 J. When light with a wavelength of 5400 Å shines on the same metal surface, the kinetic energy of ejected electrons is 1.362×10–21 J. From this information, determ ...
Physics with Negative Masses
... Translational invariance of physical laws in 4-d space-time manifests itself in the conserved energymomentum 4-vector, p = (p0, p1, p2, p3). In quantum mechanics p is an operator conjugated to the timespace position operator, x, which is expressed by the commutation relations [ x , p ]=i , ...
... Translational invariance of physical laws in 4-d space-time manifests itself in the conserved energymomentum 4-vector, p = (p0, p1, p2, p3). In quantum mechanics p is an operator conjugated to the timespace position operator, x, which is expressed by the commutation relations [ x , p ]=i , ...
Midterm Review File
... 12. The four isotopes of chromium are shown below, each with a percent abundance and the composition of its nucleus. Using these data, calculate (show work) the average atomic mass for ______________. 12 p+ 12 p+ 12 p+ 12 n0 13 n0 14 n0 ...
... 12. The four isotopes of chromium are shown below, each with a percent abundance and the composition of its nucleus. Using these data, calculate (show work) the average atomic mass for ______________. 12 p+ 12 p+ 12 p+ 12 n0 13 n0 14 n0 ...
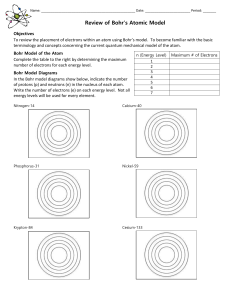
Name: ______ Date: Period: ______ Review of Bohr`s Atomic Model
... To review the placement of electrons within an atom using Bohr’s model. To become familiar with the basic terminology and concepts concerning the current quantum mechanical model of the atom. Bohr Model of the Atom ...
... To review the placement of electrons within an atom using Bohr’s model. To become familiar with the basic terminology and concepts concerning the current quantum mechanical model of the atom. Bohr Model of the Atom ...
James_Vary
... Does not follow the effective operator approach (yet) Introduce regulators as needed to obtain cutoff-independent spectra S. Dalley and B. van de Sande, Phys. Rev. D67, 114507 (2003); hep-ph/0212086 D. Chakrabarti, A. Harindranath and J.P. Vary, Phys. Rev. D69, 034502 (2004); hep-ph/0309317 ...
... Does not follow the effective operator approach (yet) Introduce regulators as needed to obtain cutoff-independent spectra S. Dalley and B. van de Sande, Phys. Rev. D67, 114507 (2003); hep-ph/0212086 D. Chakrabarti, A. Harindranath and J.P. Vary, Phys. Rev. D69, 034502 (2004); hep-ph/0309317 ...
Extension physics
... Recall that particles have wave-like nature and show how the wave nature of particles with mass can be described by Schrödinger’s Time Dependent Equation. Derive Schrödinger’s Time Independent Equation from Schrödinger’s Time Dependent Equation. Derive the solutions of an infinite potential well usi ...
... Recall that particles have wave-like nature and show how the wave nature of particles with mass can be described by Schrödinger’s Time Dependent Equation. Derive Schrödinger’s Time Independent Equation from Schrödinger’s Time Dependent Equation. Derive the solutions of an infinite potential well usi ...
Lecture 4
... Review: hydrogenic atoms (these are called ions since they are charged). A hydrogenic (or hydrogen-like) atom consists of a single electron orbiting a nucleus with Z protons. What are the corresponding energies? ...
... Review: hydrogenic atoms (these are called ions since they are charged). A hydrogenic (or hydrogen-like) atom consists of a single electron orbiting a nucleus with Z protons. What are the corresponding energies? ...
l - Evergreen
... • Rutherford observed nuclear scattering, invented orbital atom • Bohr quantized angular momentum, improved H atom model. • Bohr model explained observed H spectra, derived En = E/n2 and phenomenological Rydberg constant ...
... • Rutherford observed nuclear scattering, invented orbital atom • Bohr quantized angular momentum, improved H atom model. • Bohr model explained observed H spectra, derived En = E/n2 and phenomenological Rydberg constant ...