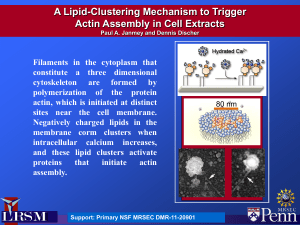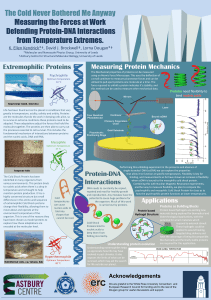
The Cold Never Bothered Me Anyway Measuring the Forces at Work
... temperature and is thought to help maintain protein production. It has a highly conserved structure but small differences in the amino acid sequence of extremophilic Cold Shock proteins change their flexibility, allowing them to move about and operate at the environment temperature of the organism. ...
... temperature and is thought to help maintain protein production. It has a highly conserved structure but small differences in the amino acid sequence of extremophilic Cold Shock proteins change their flexibility, allowing them to move about and operate at the environment temperature of the organism. ...
Diapositivo 1 - Cell Biology Promotion
... Angiogenic activity Promoting cell growth, differentiation and motility ...
... Angiogenic activity Promoting cell growth, differentiation and motility ...
Anti-GPCR GPR116 antibody ab111169 Product datasheet 1 References 2 Images
... ab111169 at 1/100 dilution staining GPCR GPR116 in A549 cells by Immunofluorescence. The image on the right ...
... ab111169 at 1/100 dilution staining GPCR GPR116 in A549 cells by Immunofluorescence. The image on the right ...
Proteins*
... Two amino acids linked together are called dipeptides More than 2 linked together are called polypeptides polypeptides can be thousands of amino acids long ...
... Two amino acids linked together are called dipeptides More than 2 linked together are called polypeptides polypeptides can be thousands of amino acids long ...
Recombinant Human PKA 2 beta (regulatory subunit) protein
... Regulatory subunit of the cAMP-dependent protein kinases involved in cAMP signaling in cells. Type II regulatory chains mediate membrane association by binding to anchoring proteins, including the MAP2 kinase. ...
... Regulatory subunit of the cAMP-dependent protein kinases involved in cAMP signaling in cells. Type II regulatory chains mediate membrane association by binding to anchoring proteins, including the MAP2 kinase. ...
Biochemistry_Summary
... Alpha helix is a secondary structure of globular proteins. alpha helices maintain their structure by forming hydrogen bonding >> so they can make no more H.bonds with the surroundings, thus they become hydrophobic >> can be inserted within the membrane. - 7TM receptors are rigid due to the extensive ...
... Alpha helix is a secondary structure of globular proteins. alpha helices maintain their structure by forming hydrogen bonding >> so they can make no more H.bonds with the surroundings, thus they become hydrophobic >> can be inserted within the membrane. - 7TM receptors are rigid due to the extensive ...
Slide 1
... PPARγ forms a heterodimer with RXR (PPARγ-RXR Complex) to bind to DNA. If ligand binds to either PPAR or RXR, changes in the heterodimer are induced which lead to the release of corepressor molecules and the recruitment of coactivator proteins resulting in the formation of a transcriptional regulato ...
... PPARγ forms a heterodimer with RXR (PPARγ-RXR Complex) to bind to DNA. If ligand binds to either PPAR or RXR, changes in the heterodimer are induced which lead to the release of corepressor molecules and the recruitment of coactivator proteins resulting in the formation of a transcriptional regulato ...
Introduction:
... Prediction of Transmembrane helices: Prediction of transmembrane helices of the olfactory receptor O2D2 was done using TMHMM v. 2.0 (Sonnhammer, et. al., 1998). It was predicted to be a seven helix bundle similar to GPCR as shown in Fig. 1A. It is interesting to note that the putative metal binding ...
... Prediction of Transmembrane helices: Prediction of transmembrane helices of the olfactory receptor O2D2 was done using TMHMM v. 2.0 (Sonnhammer, et. al., 1998). It was predicted to be a seven helix bundle similar to GPCR as shown in Fig. 1A. It is interesting to note that the putative metal binding ...
Protein misfolding associated to mild modifications of local cellular pH
... Protein misfolding associated to mild modifications of local cellular pH: Amyloid-like aggregation of human apolipoprotein A-I variants Ramella N., Tricerri M. A., Rimoldi O.J. INIBIOLP-CONICET. Fac. Ciencias Medicas, UNLP. Argentina The native folding of proteins is critical to fulfill their biolog ...
... Protein misfolding associated to mild modifications of local cellular pH: Amyloid-like aggregation of human apolipoprotein A-I variants Ramella N., Tricerri M. A., Rimoldi O.J. INIBIOLP-CONICET. Fac. Ciencias Medicas, UNLP. Argentina The native folding of proteins is critical to fulfill their biolog ...
Adenosine Transporter Receptor, human (A8352 - Sigma
... Store the product tightly sealed at –70 °C. The receptor remains active for several months when stored at –70 °C. Repeated freeze-thaw of this product is not recommended. Procedure Standard Receptor Binding Assay 1. Prepare Assay Buffer – 50 mM Tris-HCl, pH 7.4. 2. Thaw product vial quickly and mix ...
... Store the product tightly sealed at –70 °C. The receptor remains active for several months when stored at –70 °C. Repeated freeze-thaw of this product is not recommended. Procedure Standard Receptor Binding Assay 1. Prepare Assay Buffer – 50 mM Tris-HCl, pH 7.4. 2. Thaw product vial quickly and mix ...
The yellow structure represents the hydrophillic or water loving
... cell to receive instructions 1. These proteins are used in intercellular communication. 2. In this picture you can see the a hormone binding to the receptor. 3. This causes the receptor protein release a signal to perform some ...
... cell to receive instructions 1. These proteins are used in intercellular communication. 2. In this picture you can see the a hormone binding to the receptor. 3. This causes the receptor protein release a signal to perform some ...
Biozentrum: Research group Martin Spiess
... Membrane transport to the cell surface Proteins are sorted between organelles and transported in membrane vesicles. Our research focuses on the molecules and mechanisms that mediate this. In particular, we study the pathways how proteins are transported to the cell surface and secreted. ...
... Membrane transport to the cell surface Proteins are sorted between organelles and transported in membrane vesicles. Our research focuses on the molecules and mechanisms that mediate this. In particular, we study the pathways how proteins are transported to the cell surface and secreted. ...
Gene Section P2RX7 (purinergic receptor P2X, ligand-gated ion channel, 7)
... inflammatory responses. Prolonged stimulation of the P2X7 receptor can lead to plasma membrane bleb formation, opening of pannexin-1 dependent membrane pores and eventual cell death. ...
... inflammatory responses. Prolonged stimulation of the P2X7 receptor can lead to plasma membrane bleb formation, opening of pannexin-1 dependent membrane pores and eventual cell death. ...
Control of Cell Adhesion
... - ECM molecules interact with receptors, transmitting signals across the cell membrane to cytoplasmic molecules that, in turn, initiate cascades of events involving cytoskeleton and nucleus - these “nuclear events” affect specific gene expression that reciprocally regulates ECM structures and conten ...
... - ECM molecules interact with receptors, transmitting signals across the cell membrane to cytoplasmic molecules that, in turn, initiate cascades of events involving cytoskeleton and nucleus - these “nuclear events” affect specific gene expression that reciprocally regulates ECM structures and conten ...
Cell membrane structure File
... • MOST COMMON MATERIAL IN THE CELL MEMBRANE • TWO LAYERS THICK • EACH LAYER HAS A ROUNDED HEAD END (HYDROPHILIC = LOVES WATER) THAT ALWAYS FACES THE WATER BASED SOLUTION (EITHER THE CELL’S ENVIRONMENT OR THE CELL’S CYTOPLASM. • EACH PHOSPHOLIPID HAS TWO TAILS ON ONE END (HYDROPHOBIC = FEARS WATER) T ...
... • MOST COMMON MATERIAL IN THE CELL MEMBRANE • TWO LAYERS THICK • EACH LAYER HAS A ROUNDED HEAD END (HYDROPHILIC = LOVES WATER) THAT ALWAYS FACES THE WATER BASED SOLUTION (EITHER THE CELL’S ENVIRONMENT OR THE CELL’S CYTOPLASM. • EACH PHOSPHOLIPID HAS TWO TAILS ON ONE END (HYDROPHOBIC = FEARS WATER) T ...
37151
... The term ‘Proteome’ coined in 1994 Complete set of proteins exported and modified following expression, by entire genome in lifetime of a cell Proteomics is usually carried out to study the complement of protein expressed by a cell at any one time or at a particular stage ...
... The term ‘Proteome’ coined in 1994 Complete set of proteins exported and modified following expression, by entire genome in lifetime of a cell Proteomics is usually carried out to study the complement of protein expressed by a cell at any one time or at a particular stage ...
Ch. 5. Protein Purification and Characterization Techniques
... • So efficient, today N-/C-terminal residues usually not done by enzymatic/chemical cleavage ...
... • So efficient, today N-/C-terminal residues usually not done by enzymatic/chemical cleavage ...
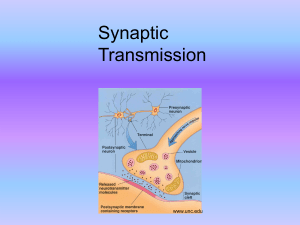
Synaptic Transmission
... • Sometimes: ion channel opens or closes • Usually: a 2nd messenger is synthesized, which can have a variety of effects (e.g. enter nucleus, bind to DNA, alter gene expression). ...
... • Sometimes: ion channel opens or closes • Usually: a 2nd messenger is synthesized, which can have a variety of effects (e.g. enter nucleus, bind to DNA, alter gene expression). ...
Protein Structure:
... The transcription factor AP1 is a heterodimer formed from the proto-oncogenes c-fos (shown in red) and c-jun (shown in blue). In order to bind to DNA, and activate transcription, the two subunits associate by virtue of hydrophobic interactions, involving a structural motif known as a leucine zipper ...
... The transcription factor AP1 is a heterodimer formed from the proto-oncogenes c-fos (shown in red) and c-jun (shown in blue). In order to bind to DNA, and activate transcription, the two subunits associate by virtue of hydrophobic interactions, involving a structural motif known as a leucine zipper ...
G protein–coupled receptor

G protein–coupled receptors (GPCRs), also known as seven-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptor, and G protein–linked receptors (GPLR), constitute a large protein family of receptors that sense molecules outside the cell and activate inside signal transduction pathways and, ultimately, cellular responses. Coupling with G proteins, they are called seven-transmembrane receptors because they pass through the cell membrane seven times.G protein–coupled receptors are found only in eukaryotes, including yeast, choanoflagellates, and animals. The ligands that bind and activate these receptors include light-sensitive compounds, odors, pheromones, hormones, and neurotransmitters, and vary in size from small molecules to peptides to large proteins. G protein–coupled receptors are involved in many diseases, and are also the target of approximately 40% of all modern medicinal drugs. Two of the United States's top five selling drugs (Hydrocodone and Lisinopril) act by targeting a G protein–coupled receptor. The 2012 Nobel Prize in Chemistry was awarded to Brian Kobilka and Robert Lefkowitz for their work that was ""crucial for understanding how G protein–coupled receptors function."". There have been at least seven other Nobel Prizes awarded for some aspect of G protein–mediated signaling.There are two principal signal transduction pathways involving the G protein–coupled receptors: the cAMP signal pathway and the phosphatidylinositol signal pathway. When a ligand binds to the GPCR it causes a conformational change in the GPCR, which allows it to act as a guanine nucleotide exchange factor (GEF). The GPCR can then activate an associated G protein by exchanging its bound GDP for a GTP. The G protein's α subunit, together with the bound GTP, can then dissociate from the β and γ subunits to further affect intracellular signaling proteins or target functional proteins directly depending on the α subunit type (Gαs, Gαi/o, Gαq/11, Gα12/13).