Carboxylic Acids and Nitriles
... Hydrolysis: Conversion of Nitriles into Carboxylic Acids A nitrile can be hydrolyzed in either basic or acidic aqueous solution to yield a carboxylic acid and ammoniz or an amine O ...
... Hydrolysis: Conversion of Nitriles into Carboxylic Acids A nitrile can be hydrolyzed in either basic or acidic aqueous solution to yield a carboxylic acid and ammoniz or an amine O ...
Stoichiometry: Predicting Amounts in Reactions
... Stoichiometry is the process of determining how much product is made or how much reactant is needed during a chemical reaction. As we know, in chemical reactions atoms are conserved. We show thi ...
... Stoichiometry is the process of determining how much product is made or how much reactant is needed during a chemical reaction. As we know, in chemical reactions atoms are conserved. We show thi ...
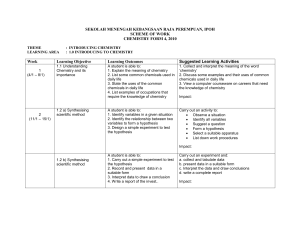
SEKOLAH MENENGAH KEBANGSAAN RAJA PEREMPUAN, IPOH
... Gather information and discuss on :a) Group 17 elements and their physical and chemical properties. b) the similarities in chemical properties of Group 17 elements c) the relationship between chemical properties of group 17 elements with their electron arrangements Carry out experiment to investigat ...
... Gather information and discuss on :a) Group 17 elements and their physical and chemical properties. b) the similarities in chemical properties of Group 17 elements c) the relationship between chemical properties of group 17 elements with their electron arrangements Carry out experiment to investigat ...
Chapter 20 Organic Chemistry
... models not only show you the attachment pattern, but give you an idea about the shape of the molecule ...
... models not only show you the attachment pattern, but give you an idea about the shape of the molecule ...
EXAM IIR - Academics
... 20. In another, parallel universe, the charge/mass ratio of a fundamental particle was measured and found to be + 5.685 x 10-12 coulombs/kg. From this one can conclude that: (A) The mass of the particle must be very large and/or the charge must be very small. (B) The particle has a net negative char ...
... 20. In another, parallel universe, the charge/mass ratio of a fundamental particle was measured and found to be + 5.685 x 10-12 coulombs/kg. From this one can conclude that: (A) The mass of the particle must be very large and/or the charge must be very small. (B) The particle has a net negative char ...
Concept Development Studies in Chemistry
... exhibit elemental iron's color, density, hardness, magnetism, etc. Since the properties of the elements are not maintained by the compound, then the compound must not be a simple mixture of the elements. We could, of course, jump directly to the answers to these questions by stating that the element ...
... exhibit elemental iron's color, density, hardness, magnetism, etc. Since the properties of the elements are not maintained by the compound, then the compound must not be a simple mixture of the elements. We could, of course, jump directly to the answers to these questions by stating that the element ...
10.3 PREPARATION OF ETHERS
... The sulfonate ester method requires two steps for the conversion of an alcohol into an alkyl chloride. A reagent that can accomplish this transformation in one step is thionyl chloride, SOCl2. In a reaction very similar to the formation of sulfonate esters, this reagent replaces the hydrogen of the ...
... The sulfonate ester method requires two steps for the conversion of an alcohol into an alkyl chloride. A reagent that can accomplish this transformation in one step is thionyl chloride, SOCl2. In a reaction very similar to the formation of sulfonate esters, this reagent replaces the hydrogen of the ...
FREE Sample Here
... A) The element may undergo radioactive decay. B) The element may react with itself and gain or lose subatomic particles. C) The atoms of the element form chemical bonds with each other, and that changes the weight of the element. D) The element may have multiple stable isotopes, and the isotopic com ...
... A) The element may undergo radioactive decay. B) The element may react with itself and gain or lose subatomic particles. C) The atoms of the element form chemical bonds with each other, and that changes the weight of the element. D) The element may have multiple stable isotopes, and the isotopic com ...
Kinetics and Mechanisms of the Reactions of Diaryl
... absorption maxima in the range 290-360 nm and decay with mixed order kinetics with concomitant formation of the corresponding digermene, Ge2R4 (R ) Me, Ph, or Mes). Absolute rate constants for formation of the complexes could be measured for GePh2 with all three substrates and for GeMe2 with THF and ...
... absorption maxima in the range 290-360 nm and decay with mixed order kinetics with concomitant formation of the corresponding digermene, Ge2R4 (R ) Me, Ph, or Mes). Absolute rate constants for formation of the complexes could be measured for GePh2 with all three substrates and for GeMe2 with THF and ...
CHM 4XX. Organometallic Chemistry (0.5
... Students who will go on to graduate school or industry will under the new program take a package of courses similar to what they would take under the current major. They will take physics for their electives and replace the two Advanced Lab courses currently required (each ½ course unit) with a choi ...
... Students who will go on to graduate school or industry will under the new program take a package of courses similar to what they would take under the current major. They will take physics for their electives and replace the two Advanced Lab courses currently required (each ½ course unit) with a choi ...
View/Open
... The rings of cycloalkenes containing five carbon atoms or fewer exist only in the cis form (Fig. 7.3). The introduction of a trans double bond into rings this small would, if it were possible, introduce greater strain than the bonds of the ring atoms could accommodate. ...
... The rings of cycloalkenes containing five carbon atoms or fewer exist only in the cis form (Fig. 7.3). The introduction of a trans double bond into rings this small would, if it were possible, introduce greater strain than the bonds of the ring atoms could accommodate. ...
C273/SQP365 NATIONAL QUALIFICATIONS Chemistry
... statement about the effect of a catalyst? The catalyst ...
... statement about the effect of a catalyst? The catalyst ...
Synthesis of enantiopure alcohols
... B from Candida antarctica (Novozym 435) and that addition of enantiopure (R)alcohols, (R)-1, (R)-2, (R)-5, (R)-6 and (R)-7, induced increase in the E-value of the esterification of 3-chloro-1-phenoxy-2-propanol (4).1 We have suggested that the increase of enantioselectivity was caused by inhibition ...
... B from Candida antarctica (Novozym 435) and that addition of enantiopure (R)alcohols, (R)-1, (R)-2, (R)-5, (R)-6 and (R)-7, induced increase in the E-value of the esterification of 3-chloro-1-phenoxy-2-propanol (4).1 We have suggested that the increase of enantioselectivity was caused by inhibition ...
equilibrium - TeacherWeb
... The direction in which you write the chemical equation for an equilibrium is arbitrary, because equilibrium can be approached from either direction. The equilibrium constant expression for a reaction written in one direction is the reciprocal of the one for the reaction in the reverse direction. The ...
... The direction in which you write the chemical equation for an equilibrium is arbitrary, because equilibrium can be approached from either direction. The equilibrium constant expression for a reaction written in one direction is the reciprocal of the one for the reaction in the reverse direction. The ...
Sample Exercise 20.1 Identifying Oxidizing and Reducing Agents
... Analyze: We are given an incomplete, unbalanced (skeleton) equation for a redox reaction occurring in acidic solution and asked to complete and balance it. Plan: We use the half-reaction procedure we just learned. Solve: Step 1: We divide the equation into two halfreactions: Step 2:We balance each h ...
... Analyze: We are given an incomplete, unbalanced (skeleton) equation for a redox reaction occurring in acidic solution and asked to complete and balance it. Plan: We use the half-reaction procedure we just learned. Solve: Step 1: We divide the equation into two halfreactions: Step 2:We balance each h ...
The structure and mass of atoms - Brentwood Ursuline Convent
... example, when methane is burned not all of it reacts to form carbon dioxide because other reactions make carbon monoxide or carbon (soot) instead. When you bake a cake, some of the ingredients get left in the bowl or on the cake tin. The same sort of thing happens in a chemical reaction. ...
... example, when methane is burned not all of it reacts to form carbon dioxide because other reactions make carbon monoxide or carbon (soot) instead. When you bake a cake, some of the ingredients get left in the bowl or on the cake tin. The same sort of thing happens in a chemical reaction. ...
Worked out problems
... Analyze: We are given an incomplete, unbalanced (skeleton) equation for a redox reaction occurring in acidic solution and asked to complete and balance it. Plan: We use the half-reaction procedure we just learned. Solve: Step 1: We divide the equation into two halfreactions: Step 2:We balance each h ...
... Analyze: We are given an incomplete, unbalanced (skeleton) equation for a redox reaction occurring in acidic solution and asked to complete and balance it. Plan: We use the half-reaction procedure we just learned. Solve: Step 1: We divide the equation into two halfreactions: Step 2:We balance each h ...
Unit 9: ACIDS AND BASES
... a. Distinguish between strong and weak acids and bases in terms of the extent of dissociation, reaction with water and electrical conductivity. b. State whether a given acid or base is strong or weak. c. Distinguish between strong and weak acids and bases, and determine the relative strengths of aci ...
... a. Distinguish between strong and weak acids and bases in terms of the extent of dissociation, reaction with water and electrical conductivity. b. State whether a given acid or base is strong or weak. c. Distinguish between strong and weak acids and bases, and determine the relative strengths of aci ...
The integration of flow reactors into synthetic organic chemistry
... tubes and Bunsen burners are all still commonly in use today despite them being invented over 160 years ago. Consequently laboratory practices have also become standardized to make the best use of these tools and associated pieces of equipment. A standard sequence for a reaction today and over a cen ...
... tubes and Bunsen burners are all still commonly in use today despite them being invented over 160 years ago. Consequently laboratory practices have also become standardized to make the best use of these tools and associated pieces of equipment. A standard sequence for a reaction today and over a cen ...
Integration of chemical catalysis with extractive fermentation to
... Using a synthetic ABE mixture of pure acetone, n-butanol and ethanol, we investigated the double alkylation of acetone to obtain heptan-4-one (B in Fig. 1) (alkylation with ethanol), nonan-4-one (D) (alkylation with one molecule each of ethanol and butanol) and undecan-6-one (F) (alkylation with but ...
... Using a synthetic ABE mixture of pure acetone, n-butanol and ethanol, we investigated the double alkylation of acetone to obtain heptan-4-one (B in Fig. 1) (alkylation with ethanol), nonan-4-one (D) (alkylation with one molecule each of ethanol and butanol) and undecan-6-one (F) (alkylation with but ...
Worked solutions to the problems
... supervision. We have also not included specific details for handling or disposal of the products of these lab exercises, as these will vary greatly from country to country, but we know that you will employ best-practice to responsibly dispose or recycle the materials that your students use and produ ...
... supervision. We have also not included specific details for handling or disposal of the products of these lab exercises, as these will vary greatly from country to country, but we know that you will employ best-practice to responsibly dispose or recycle the materials that your students use and produ ...
HALO-ORGANICS – Fully functional fluorine 1
... Other potential syntheses use trifluoroacetaldehyde or Me3Si-CF3 catalysed by TBAF. Trifluoroacetaldehyde is a very useful building block, as it can be reacted with numerous different reagents including olefins, dienes, ketene silyl acetals, metal enolates and a variety of aromatic compounds. Howeve ...
... Other potential syntheses use trifluoroacetaldehyde or Me3Si-CF3 catalysed by TBAF. Trifluoroacetaldehyde is a very useful building block, as it can be reacted with numerous different reagents including olefins, dienes, ketene silyl acetals, metal enolates and a variety of aromatic compounds. Howeve ...