Aldehydes and Ketones
... THEY HAVE LOWER BOILING POINTS THAT ALCOHOLS. • IN A SERIES OF COMPOUNDS OF SIMILAR MOLECULAR WEIGHTS, THEIR BOILING POINTS ARE AS FOLLOWS: • ALKANES < ALDEHYDES/KETONES < ALCOHOLS • ALDEHYDES AND KETONES ARE SOLUBLE IN ORGANIC SOLVENTS, AND THOSE WITH FEWER THAN FIVE CARBONS ARE ALSO SOLUBLE IN WAT ...
... THEY HAVE LOWER BOILING POINTS THAT ALCOHOLS. • IN A SERIES OF COMPOUNDS OF SIMILAR MOLECULAR WEIGHTS, THEIR BOILING POINTS ARE AS FOLLOWS: • ALKANES < ALDEHYDES/KETONES < ALCOHOLS • ALDEHYDES AND KETONES ARE SOLUBLE IN ORGANIC SOLVENTS, AND THOSE WITH FEWER THAN FIVE CARBONS ARE ALSO SOLUBLE IN WAT ...
IOSR Journal of Applied Chemistry (IOSR-JAC)
... Reduction of aromatic nitro compounds to corresponding amines is an extensively studied organic transformation [1]. Diverse reagents and reaction conditions have been developed for this purpose. Conversion of aromatic amines to corresponding acetamides is also well documented [2]. Reduction of nitro ...
... Reduction of aromatic nitro compounds to corresponding amines is an extensively studied organic transformation [1]. Diverse reagents and reaction conditions have been developed for this purpose. Conversion of aromatic amines to corresponding acetamides is also well documented [2]. Reduction of nitro ...
Photoelectron spectroscopy of chromium
... their findings. The excellent agreement between these measured and calculated electronic properties suggests that the calculated structural and magnetic results are also correct. Negative ion photoelectron 共photodetachment兲 spectroscopy is conducted by crossing a mass-selected beam of anions with a ...
... their findings. The excellent agreement between these measured and calculated electronic properties suggests that the calculated structural and magnetic results are also correct. Negative ion photoelectron 共photodetachment兲 spectroscopy is conducted by crossing a mass-selected beam of anions with a ...
- TestbankU
... 39. The condensed structure for dimethyl ether looks symmetrical. However, dimethyl ether has a dipole moment. Draw a structure that explains this and indicate the expected direction of the molecular dipole moment. ...
... 39. The condensed structure for dimethyl ether looks symmetrical. However, dimethyl ether has a dipole moment. Draw a structure that explains this and indicate the expected direction of the molecular dipole moment. ...
Protonation patterns in reduced and oxidized forms of electron
... The atomistic details of many biological processes provide deeper insight in the mechanism of their work. Techniques such as X-ray crystallography and NMR are a powerful tools for atomic resolution structure determination of biomolecules. However, they produce limited number of molecular structures ...
... The atomistic details of many biological processes provide deeper insight in the mechanism of their work. Techniques such as X-ray crystallography and NMR are a powerful tools for atomic resolution structure determination of biomolecules. However, they produce limited number of molecular structures ...
Chapter 13 PowerPoint
... Once equilibrium is achieved, the amount of each reactant and product remains constant. ...
... Once equilibrium is achieved, the amount of each reactant and product remains constant. ...
Rutgers...Ch17 Reactions of Aromatic Compounds
... The enhanced rate and substitution pattern for toluene can be explained by considering the structures of the intermediate sigma complexes for substitution at each of the different positions. The RDS is highly endothermic, therefore according to Hammond's postulate (Ch 4), the energy of the TS should ...
... The enhanced rate and substitution pattern for toluene can be explained by considering the structures of the intermediate sigma complexes for substitution at each of the different positions. The RDS is highly endothermic, therefore according to Hammond's postulate (Ch 4), the energy of the TS should ...
Amine-functionalized boehmite nanoparticle-supported
... tion progress was monitored by GLC. Because different alkenes have different reactivities toward oxidation, these reactions were continued until no further progress was observed. The ...
... tion progress was monitored by GLC. Because different alkenes have different reactivities toward oxidation, these reactions were continued until no further progress was observed. The ...
Effect of N-donor ancillary ligands on structural and magnetic
... structural diversity and luminescent properties. The combination of the characteristics of the organic and inorganic components allows us to obtain new materials with potential applications in catalysis, separation, sorption, luminescence, biological chemistry, etc.1 The common strategy for synthesi ...
... structural diversity and luminescent properties. The combination of the characteristics of the organic and inorganic components allows us to obtain new materials with potential applications in catalysis, separation, sorption, luminescence, biological chemistry, etc.1 The common strategy for synthesi ...
Chapter 8 Quantities in Chemical Reactions
... • MTBE made its way into drinking water through gasoline spills at gas stations, from boat motors, and from leaking underground storage tanks. • Ethanol (C2H5OH), made from the fermentation of grains, is now used as a substitute for MTBE to increase oxygen content in motor fuel. • Ethanol was not us ...
... • MTBE made its way into drinking water through gasoline spills at gas stations, from boat motors, and from leaking underground storage tanks. • Ethanol (C2H5OH), made from the fermentation of grains, is now used as a substitute for MTBE to increase oxygen content in motor fuel. • Ethanol was not us ...
Chemistry Unit Outcomes
... Explain what knowing the number of outer shell electron in an element allows you to predict. Describe how a chemical bond between 2 atoms forms. Explain the meaning of what is known as a valence shell and valence electrons. Outline to what chemical properties of elements are related. Describe what i ...
... Explain what knowing the number of outer shell electron in an element allows you to predict. Describe how a chemical bond between 2 atoms forms. Explain the meaning of what is known as a valence shell and valence electrons. Outline to what chemical properties of elements are related. Describe what i ...
LABORATORY MANUAL CHEMISTRY 121
... the rate law and rate constants for this reaction, we shall measure the half-life for each experiment. When 50% of the reactant has been converted to product, the mixture (50% green and 50% red) has a characteristic color best described as "gun-metal gray", but other colors are possible and your ins ...
... the rate law and rate constants for this reaction, we shall measure the half-life for each experiment. When 50% of the reactant has been converted to product, the mixture (50% green and 50% red) has a characteristic color best described as "gun-metal gray", but other colors are possible and your ins ...
1. What energy changes occur when chemical bonds are formed
... The reaction is spontaneous at low temperatures but becomes non-spontaneous at high temperatures. ...
... The reaction is spontaneous at low temperatures but becomes non-spontaneous at high temperatures. ...
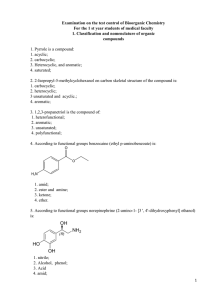
2. 2-Isopropyl-5-methylcyclohexanol on carbon skeletal
... 23. Energy of 2-chlorobutane in the eclipsed conformation more than gauche because in the eclipsed conformation: 1. The other configuration of the molecule; 2. The greater torsional stress; 3. increased Van der Waals repulsion. 4. the molecule another electronic structure; 24. The conformations of 1 ...
... 23. Energy of 2-chlorobutane in the eclipsed conformation more than gauche because in the eclipsed conformation: 1. The other configuration of the molecule; 2. The greater torsional stress; 3. increased Van der Waals repulsion. 4. the molecule another electronic structure; 24. The conformations of 1 ...
Document
... Heat, q The energy that flows into or out of a system because of a difference in temperature between the thermodynamic system and its surroundings Heat flows spontaneously from a region of higher temperature to a region of lower temperature. • q is defined as positive if heat is absorbed by the sys ...
... Heat, q The energy that flows into or out of a system because of a difference in temperature between the thermodynamic system and its surroundings Heat flows spontaneously from a region of higher temperature to a region of lower temperature. • q is defined as positive if heat is absorbed by the sys ...
Minimum electrophilicity principle in Lewis acid–base complexes of
... with these acids, are considered here. It is expected that more stable complexes are formed by stronger acids. Therefore, according to the MHP and MEP, for each set of complexes which are formed for a given base and different acids, the compound with the higher hardness or lesser electrophilicity be ...
... with these acids, are considered here. It is expected that more stable complexes are formed by stronger acids. Therefore, according to the MHP and MEP, for each set of complexes which are formed for a given base and different acids, the compound with the higher hardness or lesser electrophilicity be ...
4 Organic Chemistry
... these two compounds by giving them different names. In this case it is quite straightforward. We call the straight-chain molecule n-butane and the branched molecule iso-butane. However, when alkanes have more than one branch (as many do) we really do need a systematic way of naming them. Rule 1: Cho ...
... these two compounds by giving them different names. In this case it is quite straightforward. We call the straight-chain molecule n-butane and the branched molecule iso-butane. However, when alkanes have more than one branch (as many do) we really do need a systematic way of naming them. Rule 1: Cho ...
atomic and molecular physics using positron traps
... positron trapping. In order to reduce the density of impurity molecules in the system (base pressure ≤ 1 x 10 -9 torr), a cryosurface was placed in situ in the vacuum chamber as necessary. It was cooled with either liquid nitrogen (to 77K) or with an ethanol-water mixture (to ~ 266 K), depending on ...
... positron trapping. In order to reduce the density of impurity molecules in the system (base pressure ≤ 1 x 10 -9 torr), a cryosurface was placed in situ in the vacuum chamber as necessary. It was cooled with either liquid nitrogen (to 77K) or with an ethanol-water mixture (to ~ 266 K), depending on ...