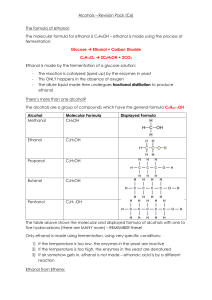
Reprinted from The Journal of Physical Chemistry, lgg5, 99
... are far lower than the energy difference between the highest occupiedmolecular orbitals(HOMO) and the lowest unoccupied molecular orbital (LUMO) of the adsorbed molecules. Both experimental and theoretical work suggeststhat the STM images at thesebias voltages do not corresponddirectly to the electr ...
... are far lower than the energy difference between the highest occupiedmolecular orbitals(HOMO) and the lowest unoccupied molecular orbital (LUMO) of the adsorbed molecules. Both experimental and theoretical work suggeststhat the STM images at thesebias voltages do not corresponddirectly to the electr ...
Organic Chemistry II Introduction
... • Alkylation occurs when the nucleophilic enolate ion reacts with the electrophilic alkyl halide or tosylate and displaces the leaving group ...
... • Alkylation occurs when the nucleophilic enolate ion reacts with the electrophilic alkyl halide or tosylate and displaces the leaving group ...
Asymmetric Catalytic Aldol
... plants and activate the donor by forming a schiff base as an intermediate. • Type II aldolases are found in bacteria and fungi and contain a Zn2+ cofactor in the active site. • In both types of aldolases the formation of the enolate is rate determining. • These enzymes generally tolerate a broad ran ...
... plants and activate the donor by forming a schiff base as an intermediate. • Type II aldolases are found in bacteria and fungi and contain a Zn2+ cofactor in the active site. • In both types of aldolases the formation of the enolate is rate determining. • These enzymes generally tolerate a broad ran ...
1.02 x 10 = 3 mol lit 3.4 x 10
... The colour of halogens is due to the fact that their molecules absorb radiations from visible light and the outer electrons are easily excited to higher energy levels. The amount of energy required for excitation depends upon the size of the atom. Fluorine atom is the smallest and the force of attra ...
... The colour of halogens is due to the fact that their molecules absorb radiations from visible light and the outer electrons are easily excited to higher energy levels. The amount of energy required for excitation depends upon the size of the atom. Fluorine atom is the smallest and the force of attra ...
Chemistry - WorkNotes
... metals and transition metals, trends in ionization energy, electronegativity, and the relative sizes of ions and atoms. ...
... metals and transition metals, trends in ionization energy, electronegativity, and the relative sizes of ions and atoms. ...
Organic Chemistry
... Multiple bonds affect, physical and chemical properties (reactivity of organic molecules). ...
... Multiple bonds affect, physical and chemical properties (reactivity of organic molecules). ...
CHEMISTRY B- MOLES PACKET NAME: HR: ______ PAGE 1
... We are about to start on a unit of chemical calculations called “stoichiometry”. Stoichiometry is how we calculate the relationships between the amounts of reactants and the amounts of products. For example, if we know the amount of reactants we have, we can use stoichiometry to calculate how many p ...
... We are about to start on a unit of chemical calculations called “stoichiometry”. Stoichiometry is how we calculate the relationships between the amounts of reactants and the amounts of products. For example, if we know the amount of reactants we have, we can use stoichiometry to calculate how many p ...
74 CHAPTER-IV "LEAD (IV) ACETATE OXIDATIONS"
... A survey of the literature revealed that a successful attempt at 1, 2-carbonyl transposition was simultaneously reported by Perkin48 and Bredt49 in 1911. Since then other methods were developed dealing with 1,2-carbonyl transposition in the terpene systems50' 51 and steroids systems. In 1944, Ruzika ...
... A survey of the literature revealed that a successful attempt at 1, 2-carbonyl transposition was simultaneously reported by Perkin48 and Bredt49 in 1911. Since then other methods were developed dealing with 1,2-carbonyl transposition in the terpene systems50' 51 and steroids systems. In 1944, Ruzika ...
2 - Scheikundeolympiade
... The official English version of this examination is available on request only for ...
... The official English version of this examination is available on request only for ...
FREE Sample Here
... A) The element may undergo radioactive decay. B) The element may react with itself and gain or lose subatomic particles. C) The atoms of the element form chemical bonds with each other, and that changes the weight of the element. D) The element may have multiple stable isotopes, and the isotopic com ...
... A) The element may undergo radioactive decay. B) The element may react with itself and gain or lose subatomic particles. C) The atoms of the element form chemical bonds with each other, and that changes the weight of the element. D) The element may have multiple stable isotopes, and the isotopic com ...
Document
... If it smells of “fish” or “rotting flesh” chances are you have an amine!! Amines behave in a similar way to NH3 but their behaviour is modified by the alkyl groups. 2.1 Bonding: cf NH3 ...
... If it smells of “fish” or “rotting flesh” chances are you have an amine!! Amines behave in a similar way to NH3 but their behaviour is modified by the alkyl groups. 2.1 Bonding: cf NH3 ...