Organic and Inorganic Esters from Alcohols
... crown ether 18-crown-6 contains 18 total atoms and 6 oxygen atoms. ...
... crown ether 18-crown-6 contains 18 total atoms and 6 oxygen atoms. ...
02-Atoms-Molecules
... Endergonic reactions – reactions whose products contain more potential energy than did its reactants ...
... Endergonic reactions – reactions whose products contain more potential energy than did its reactants ...
Packet
... 29. An element a. can be broken down into simpler substances b. are used to make other elements c. are used to make compounds d. are never found in the periodic table of elements 30. Physical means can be used to separate a. elements b. pure substances b. mixtures d. compounds 31. Anything that take ...
... 29. An element a. can be broken down into simpler substances b. are used to make other elements c. are used to make compounds d. are never found in the periodic table of elements 30. Physical means can be used to separate a. elements b. pure substances b. mixtures d. compounds 31. Anything that take ...
Novascan PSD-UVT Decontamination System
... The PSD‐UVT was designed for applications in the electronic, semiconductor and scientific industries. A mercury vapor lamp generates ultra‐violet light and ozone resulting in the atomic cleaning of silicon, silicon nitride, glass and other materials. The PSD‐UVT can also be used for UV curing, UV ...
... The PSD‐UVT was designed for applications in the electronic, semiconductor and scientific industries. A mercury vapor lamp generates ultra‐violet light and ozone resulting in the atomic cleaning of silicon, silicon nitride, glass and other materials. The PSD‐UVT can also be used for UV curing, UV ...
Abstract
... to include all three spin components ( = 4, 3, and 2). CoF was created by reacting cobalt vapor with a mixture of 10% F in He. Fourteen rotational transitions were recorded. -doubling was observed in both the = 3 (5 MHz separation) and = 2 (100 MHz) spin components, an unexpected result fo ...
... to include all three spin components ( = 4, 3, and 2). CoF was created by reacting cobalt vapor with a mixture of 10% F in He. Fourteen rotational transitions were recorded. -doubling was observed in both the = 3 (5 MHz separation) and = 2 (100 MHz) spin components, an unexpected result fo ...
Chapter One Outline
... Identifying Matter: Physical Properties Physical properties can be observed and measured without changing the composition of a substance. Examples include temperature, mass, density, etc. Density is the ratio of an objects mass to its volume; D = m/v Chemical Properties A substances chemical propert ...
... Identifying Matter: Physical Properties Physical properties can be observed and measured without changing the composition of a substance. Examples include temperature, mass, density, etc. Density is the ratio of an objects mass to its volume; D = m/v Chemical Properties A substances chemical propert ...
Chapter 12 Alcohols, Phenols, Ethers, Aldehydes, and Ketones
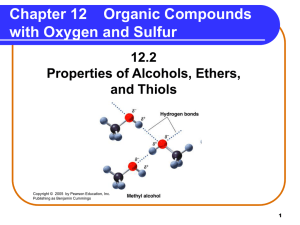
... groups. • form hydrogen bonds with other alcohol molecules. • have higher boiling points than alkanes and ethers of similar mass. ...
... groups. • form hydrogen bonds with other alcohol molecules. • have higher boiling points than alkanes and ethers of similar mass. ...
CSUS Department of Chemistry Molecular Shapes Chem. 1A Page
... ethyl alcohol and structure 6 is dimethyl ether.) None of the atoms in structure 7 has a formal charge, but in structure 8 the oxygen atom has a −1 formal charge and the double‐bonded chlorine atom has a +1 formal charge. In most cases, structures without formal charges are more stable, so we pre ...
... ethyl alcohol and structure 6 is dimethyl ether.) None of the atoms in structure 7 has a formal charge, but in structure 8 the oxygen atom has a −1 formal charge and the double‐bonded chlorine atom has a +1 formal charge. In most cases, structures without formal charges are more stable, so we pre ...
1/4 1. ATP synthesis How much energy is stored in bringing together
... A two-dimensional 1H-15N correlation spectrum has been obtained by transferring magnetization from amide proton to amide nitrogen to improve signal-to-noise ratio. Subsequently the frequency of nitrogen (fN) has been collected and thereafter magnetization has been transferred back to amide proton fo ...
... A two-dimensional 1H-15N correlation spectrum has been obtained by transferring magnetization from amide proton to amide nitrogen to improve signal-to-noise ratio. Subsequently the frequency of nitrogen (fN) has been collected and thereafter magnetization has been transferred back to amide proton fo ...
CHEM 482: Physical Chemistry II
... Development of Quantum Mechanics Chapter 1 in Atkins’ Physical Chemistry Chapters 1 & 2 in McMahon’s Quantum Mechanics Demystified ...
... Development of Quantum Mechanics Chapter 1 in Atkins’ Physical Chemistry Chapters 1 & 2 in McMahon’s Quantum Mechanics Demystified ...
ChemicalBondingTestAnswers
... 1. Bohr’s model of the atom includes neutrons located in energy levels. False Correct statement: Bohr’s model of the atom includes electrons located in energy levels. 2. The noble gases exist as individual atom and are quite reactive. False Correct statement: The noble gases exist as individual atom ...
... 1. Bohr’s model of the atom includes neutrons located in energy levels. False Correct statement: Bohr’s model of the atom includes electrons located in energy levels. 2. The noble gases exist as individual atom and are quite reactive. False Correct statement: The noble gases exist as individual atom ...
Simple Chemical Reactions
... 40 cm3 Industrial denatured alcohol (IDA is highly flammable) If you are planning on using alternative fuels contact SSERC first for advice. ...
... 40 cm3 Industrial denatured alcohol (IDA is highly flammable) If you are planning on using alternative fuels contact SSERC first for advice. ...
Syllabus - York College of Pennsylvania
... It is the overall intention of this course for students to learn how to apply concepts they learn to new situations and problems by forming effective problem-solving strategies. Additionally, students who successfully complete this course should be able to: 1. Draw organic structures and depict thei ...
... It is the overall intention of this course for students to learn how to apply concepts they learn to new situations and problems by forming effective problem-solving strategies. Additionally, students who successfully complete this course should be able to: 1. Draw organic structures and depict thei ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 14. Explain with an example: (a) Bohr's Correspondence Principle (b) BornOppenheimer Approximation 15. In solving the H2+ problem using the LCAO method, the lowest energy obtained is ...
... 14. Explain with an example: (a) Bohr's Correspondence Principle (b) BornOppenheimer Approximation 15. In solving the H2+ problem using the LCAO method, the lowest energy obtained is ...
carbon and molecular diversity
... ________________________ = Molecules that contain carbon B. Carbon atoms are the most versatile building blocks of molecules The carbon atom: - Usually has an atomic number of 6; therefore, it has ____ valence electrons. - Usually completes its outer energy shell by ____________ valence electrons in ...
... ________________________ = Molecules that contain carbon B. Carbon atoms are the most versatile building blocks of molecules The carbon atom: - Usually has an atomic number of 6; therefore, it has ____ valence electrons. - Usually completes its outer energy shell by ____________ valence electrons in ...
Chemistry COS 2011-2012
... The properties of gasses can be determined through a combination of equations derived from the ideal gas law. Diffusion is the gradual mixing of two substances due to the spontaneous random motion of their particles. Effusion is the process of a molecule randomly passing through a tiny opening in a ...
... The properties of gasses can be determined through a combination of equations derived from the ideal gas law. Diffusion is the gradual mixing of two substances due to the spontaneous random motion of their particles. Effusion is the process of a molecule randomly passing through a tiny opening in a ...