study guide and review for first semester final
... 22. Using specific heat and heat capacity data as well as temperature changes that occur in a calorimeter, calculate the heat of a reaction. Ex. In a calorimeter containing 100 g of water, a reaction caused the temperature to rise 15.0 oC. How many Joules were given off? Convert the value to calori ...
... 22. Using specific heat and heat capacity data as well as temperature changes that occur in a calorimeter, calculate the heat of a reaction. Ex. In a calorimeter containing 100 g of water, a reaction caused the temperature to rise 15.0 oC. How many Joules were given off? Convert the value to calori ...
Resource for Final Exam Prep
... physical states of elements (gas, liquid, solid, monoatomic/diatomic etc). Know equations for general reactions such as the following: For active metals: metal + water metal hydroxide + H2(g) Metal oxide + water metal hydroxide (aq) [basic solution] metal oxide + acid salt + H2O Nonmetal oxide ...
... physical states of elements (gas, liquid, solid, monoatomic/diatomic etc). Know equations for general reactions such as the following: For active metals: metal + water metal hydroxide + H2(g) Metal oxide + water metal hydroxide (aq) [basic solution] metal oxide + acid salt + H2O Nonmetal oxide ...
Bacteria and Virus Research Jigsaw
... CHEMICAL EQUATION CHEMICAL EQUATION IS AN EASIER AND SHORTER WAY TO WRITE A CHEMICAL REACTION USING CHEMICAL SYMBOLS AND FORMULAS AS A SHORTCUT TO DESCRIBE A CHEMICAL REACTION ...
... CHEMICAL EQUATION CHEMICAL EQUATION IS AN EASIER AND SHORTER WAY TO WRITE A CHEMICAL REACTION USING CHEMICAL SYMBOLS AND FORMULAS AS A SHORTCUT TO DESCRIBE A CHEMICAL REACTION ...
- Aboriginal Access to Engineering
... process and reduces fever, swelling and pain. Willow bark tea does exactly the same thing because boiling the bark in water releases salicin. The healing properties of willow bark have been known to the Chinese and Aboriginal peoples for thousands of years; they were only recognized by western scien ...
... process and reduces fever, swelling and pain. Willow bark tea does exactly the same thing because boiling the bark in water releases salicin. The healing properties of willow bark have been known to the Chinese and Aboriginal peoples for thousands of years; they were only recognized by western scien ...
Redox reactions
... forming carbon dioxide and water • The structure of the compounds’ molecules is completely destroyed, with the carbon and hydrogen atoms in each molecule being oxidised • Combustion is exothermic, and ethanol is used as a fuel where it can be produced cheaply ...
... forming carbon dioxide and water • The structure of the compounds’ molecules is completely destroyed, with the carbon and hydrogen atoms in each molecule being oxidised • Combustion is exothermic, and ethanol is used as a fuel where it can be produced cheaply ...
Ionic And Covalent Bonds
... f. Be able to count the number of protons, neutrons, and electrons in an atom, ion or isotope. ...
... f. Be able to count the number of protons, neutrons, and electrons in an atom, ion or isotope. ...
Chemistry 101 H Introduction to Organic Chemistry Chapter 6
... are the first 3 members of the alkane family. All alkanes have the general formula CnH2n+2 ...
... are the first 3 members of the alkane family. All alkanes have the general formula CnH2n+2 ...
ppt - Erice Crystallography 2004
... “…the synthetic chemist is likely to have many opportunities to encounter unusual phenemona by accident during everyday chemical work with crystalline solids and without the proper background will not be prepared to recognize and take advantage of such chance discoveries. There is the further misfor ...
... “…the synthetic chemist is likely to have many opportunities to encounter unusual phenemona by accident during everyday chemical work with crystalline solids and without the proper background will not be prepared to recognize and take advantage of such chance discoveries. There is the further misfor ...
Physical and Chemical change: Introduction
... During a chemical change, the particles themselves are changed in some way. In the example of copper (II) chloride that was used earlier, the CuCl2 molecules were split up into their component atoms. The number of particles will change because each CuCl2 molecule breaks down into one copper atom (Cu ...
... During a chemical change, the particles themselves are changed in some way. In the example of copper (II) chloride that was used earlier, the CuCl2 molecules were split up into their component atoms. The number of particles will change because each CuCl2 molecule breaks down into one copper atom (Cu ...
MYP Chemistry: Final Review
... How are elements in the same group (column) related? How are the alkali metals all related? The noble gases? All have the same final electron configuration number; all have same number of valence electrons Alkali Metals: end in s1 configuration, have 1 valence electron Noble gases: end in s2p6, have ...
... How are elements in the same group (column) related? How are the alkali metals all related? The noble gases? All have the same final electron configuration number; all have same number of valence electrons Alkali Metals: end in s1 configuration, have 1 valence electron Noble gases: end in s2p6, have ...
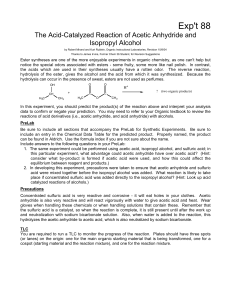
The Acid-Catalyzed Reaction of Acetic
... Using a glass Pasteur pipet, cautiously add 3 drops of concentrated sulfuric acid, one drop at a time, letting the sulfuric acid run down the inside wall of the flask neck. Add 4 boiling chips and gently swirl the liquids so that they are thoroughly mixed. Now slowly add 1.3 mL of isopropyl alcohol. ...
... Using a glass Pasteur pipet, cautiously add 3 drops of concentrated sulfuric acid, one drop at a time, letting the sulfuric acid run down the inside wall of the flask neck. Add 4 boiling chips and gently swirl the liquids so that they are thoroughly mixed. Now slowly add 1.3 mL of isopropyl alcohol. ...
Covalent Bonds - WordPress.com
... the forward reaction become reactants for the reverse reaction • Chemical equilibrium is reached when the forward and reverse reaction rates are equal ...
... the forward reaction become reactants for the reverse reaction • Chemical equilibrium is reached when the forward and reverse reaction rates are equal ...
T. V. RajanBabu Chemistry, 730 Autumn 1997
... Axial vs equatorial approach to cyclic carbonyl compounds by nucleophiles Klein / Cieplak models Tortional interactions in bicyclic systems Ring closure and ring size (Baldwin’s rules) - enthalpy and entropy of activation Bürgi-Dunitz angle, Radical cyclization reactions under kinetic vs thermodynam ...
... Axial vs equatorial approach to cyclic carbonyl compounds by nucleophiles Klein / Cieplak models Tortional interactions in bicyclic systems Ring closure and ring size (Baldwin’s rules) - enthalpy and entropy of activation Bürgi-Dunitz angle, Radical cyclization reactions under kinetic vs thermodynam ...
730-2005 topics
... Axial vs equatorial approach to cyclic carbonyl compounds by nucleophiles Klein / Cieplak models Tortional interactions in bicyclic systems Ring closure and ring size (Baldwin’s rules) - enthalpy and entropy of activation Bürgi-Dunitz angle, Radical cyclization reactions under kinetic vs thermodynam ...
... Axial vs equatorial approach to cyclic carbonyl compounds by nucleophiles Klein / Cieplak models Tortional interactions in bicyclic systems Ring closure and ring size (Baldwin’s rules) - enthalpy and entropy of activation Bürgi-Dunitz angle, Radical cyclization reactions under kinetic vs thermodynam ...
KFUPM
... What types of polysaccharides are present in cotton? In which other plants these polysaccharides are also present? Give the structure and the characteristics of these polysaccharides. Cellulose, which is the most abundant type of polysaccharides. It exits in the cell wall of green plants, for exampl ...
... What types of polysaccharides are present in cotton? In which other plants these polysaccharides are also present? Give the structure and the characteristics of these polysaccharides. Cellulose, which is the most abundant type of polysaccharides. It exits in the cell wall of green plants, for exampl ...
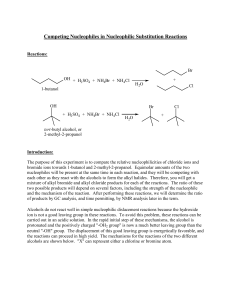
Competing Nucleophiles in Nucleophilic Substitution Reactions
... The sulfuric acid, ammonium bromide, and ammonium chloride will be provided to you as a solvent-nucleophile medium. One mL of this solution contains 0.42 mL of sulfuric acid, 0.1056 g of ammonium chloride, and 0.1944 g of ammonium bromide. From this information, you will be able to calculate the act ...
... The sulfuric acid, ammonium bromide, and ammonium chloride will be provided to you as a solvent-nucleophile medium. One mL of this solution contains 0.42 mL of sulfuric acid, 0.1056 g of ammonium chloride, and 0.1944 g of ammonium bromide. From this information, you will be able to calculate the act ...