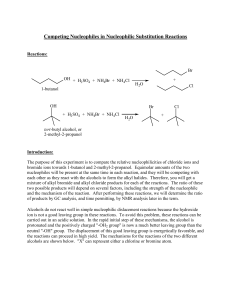
Competing Nucleophiles in Nucleophilic Substitution Reactions
... The sulfuric acid, ammonium bromide, and ammonium chloride will be provided to you as a solvent-nucleophile medium. One mL of this solution contains 0.42 mL of sulfuric acid, 0.1056 g of ammonium chloride, and 0.1944 g of ammonium bromide. From this information, you will be able to calculate the act ...
... The sulfuric acid, ammonium bromide, and ammonium chloride will be provided to you as a solvent-nucleophile medium. One mL of this solution contains 0.42 mL of sulfuric acid, 0.1056 g of ammonium chloride, and 0.1944 g of ammonium bromide. From this information, you will be able to calculate the act ...
Lab No - Scarsdale Schools
... Short, wooden pegs, long wooden pegs, and springs are used to connect the atoms in the formation of molecules of compounds and represent chemical bonds. Each chemical bond in the models represents a pair of electrons shared by two bonded atoms. This type of chemical bond is called a covalent bond. ...
... Short, wooden pegs, long wooden pegs, and springs are used to connect the atoms in the formation of molecules of compounds and represent chemical bonds. Each chemical bond in the models represents a pair of electrons shared by two bonded atoms. This type of chemical bond is called a covalent bond. ...
Introduction to Computational Chemistry
... Closely related to the desire for qualitative understanding is the nature of developing models of reality. A model aims at providing a conceptual framework in which a restricted part of reality can be ...
... Closely related to the desire for qualitative understanding is the nature of developing models of reality. A model aims at providing a conceptual framework in which a restricted part of reality can be ...
CHEM 462 Inorganic/Organometallic Chemistry Fall 2016 Midterm
... neutral, 2-electron donor; anionic, 2-electron donor; dianionic, 4-electron donor; neutral, 4-electron donor. c) According to your assignment for the divalent carbon ligand, give the oxidation state of the metal and the d-electron count in each case. d) Which divalent carbon ligand is most likely to ...
... neutral, 2-electron donor; anionic, 2-electron donor; dianionic, 4-electron donor; neutral, 4-electron donor. c) According to your assignment for the divalent carbon ligand, give the oxidation state of the metal and the d-electron count in each case. d) Which divalent carbon ligand is most likely to ...
mechanisms - Manasquan Public Schools
... O3 + NO reaction occurs in a single ELEMENTARY step. Most others involve a sequence of elementary steps. Adding elementary steps gives NET reaction. ...
... O3 + NO reaction occurs in a single ELEMENTARY step. Most others involve a sequence of elementary steps. Adding elementary steps gives NET reaction. ...
الشريحة 1 - Systematic Approach to Teaching
... In studying classes of organic chemistry, if we do the study for each of the function groups separately without making the relation between them, this will be the linear approach of teaching. ...
... In studying classes of organic chemistry, if we do the study for each of the function groups separately without making the relation between them, this will be the linear approach of teaching. ...
Carbon and the Molecular Diversity of Life
... Carbon has little tendency to gain or lose electrons. It has a valence number of 4 and forms four covalent bonds. Each carbon atom in a carbon compound is an intersection point and so a molecule can branch off in four directions. This makes it TETRAVALENT. Single covalent bonds form a tetrahedron li ...
... Carbon has little tendency to gain or lose electrons. It has a valence number of 4 and forms four covalent bonds. Each carbon atom in a carbon compound is an intersection point and so a molecule can branch off in four directions. This makes it TETRAVALENT. Single covalent bonds form a tetrahedron li ...
Introduction to Oil Chemistry and Transesterification
... atoms to exist in a lower energy state than the previous configuration. Spontaneous reactions release energy. Often times a chemical reaction may not result in a significant change in energy state, these are called reversible reactions. Example: Sodium carbonate formation ...
... atoms to exist in a lower energy state than the previous configuration. Spontaneous reactions release energy. Often times a chemical reaction may not result in a significant change in energy state, these are called reversible reactions. Example: Sodium carbonate formation ...
Academic Chemistry Final Exam Review
... 3. Describe what happens when a compound undergoes a change in physical state (from solid to liquid). ...
... 3. Describe what happens when a compound undergoes a change in physical state (from solid to liquid). ...
EXPLORING ORGANIC CHEMISTRY FOR TEACHERS (CHMY 591
... Organic chemistry is an organized study of the myriad ways that carbon compounds form and interact. These interactions are often familiar to us in everyday applications. Indeed, it is often said that life on this planet is carbon-based. The intent of our course is to familiarize you with how the att ...
... Organic chemistry is an organized study of the myriad ways that carbon compounds form and interact. These interactions are often familiar to us in everyday applications. Indeed, it is often said that life on this planet is carbon-based. The intent of our course is to familiarize you with how the att ...
01. Structure and properties of organic compounds. Aldehydes fnd
... Addition reactions brought about by nucleophiles are called nucleophilic addition reactions: Addition reactions brought about by electrophiles are called electrophilic addition reactions. Addition reactions brought about by free radicals are called free radical addition reactions. ...
... Addition reactions brought about by nucleophiles are called nucleophilic addition reactions: Addition reactions brought about by electrophiles are called electrophilic addition reactions. Addition reactions brought about by free radicals are called free radical addition reactions. ...
Review (Chapter 1) 1) Indicate if the following statements are true
... ) Bromine is more electronegative than chlorine. ) Nitrogen is less electronegative than oxygen. ) The valence of silicon is 4 ) Sodium is more electropositive than lithium. ) increasing atomic size increases the electropositivity of metals. ) Polarity of bonds depends on their atonmic size. ) Polar ...
... ) Bromine is more electronegative than chlorine. ) Nitrogen is less electronegative than oxygen. ) The valence of silicon is 4 ) Sodium is more electropositive than lithium. ) increasing atomic size increases the electropositivity of metals. ) Polarity of bonds depends on their atonmic size. ) Polar ...
Document
... (c) Goggles must be worn only when dealing with chemicals. (d) If you break a piece of glassware, you should pick up all the pieces with your hands. ...
... (c) Goggles must be worn only when dealing with chemicals. (d) If you break a piece of glassware, you should pick up all the pieces with your hands. ...
You Light Up My Life
... ◦ Molecules are mirror images of each other ◦ Cells can tell the two apart ◦ Usually one is biologically active while the other is ...
... ◦ Molecules are mirror images of each other ◦ Cells can tell the two apart ◦ Usually one is biologically active while the other is ...
Biology: Concepts and Connections, 6e (Campbell)
... 31) An oil may be converted into a substance that is solid at room temperature by A) adding hydrogens, decreasing the number of double bonds in the molecules. 32) A diet high in animal products and hydrogenated vegetable margarine may increase the risk for atherosclerosis. This is because D) most an ...
... 31) An oil may be converted into a substance that is solid at room temperature by A) adding hydrogens, decreasing the number of double bonds in the molecules. 32) A diet high in animal products and hydrogenated vegetable margarine may increase the risk for atherosclerosis. This is because D) most an ...
Biology: Concepts and Connections, 6e (Campbell)
... An oil may be converted into a substance that is solid at room temperature by A) adding hydrogens, decreasing the number of double bonds in the molecules. 32) A diet high in animal products and hydrogenated vegetable margarine may increase the risk for atherosclerosis. This is because D) most animal ...
... An oil may be converted into a substance that is solid at room temperature by A) adding hydrogens, decreasing the number of double bonds in the molecules. 32) A diet high in animal products and hydrogenated vegetable margarine may increase the risk for atherosclerosis. This is because D) most animal ...
Chapter 4 The Importance of Carbon
... Each protein has a unique, defined amino acid sequence The Shape of Globular Proteins Globular protein chains are folded up into complex shapes Examine three dimensional structure with X-ray diffraction Myoglobin first one examined All internal amino acids are nonpolar Hydrophobic interactions shove ...
... Each protein has a unique, defined amino acid sequence The Shape of Globular Proteins Globular protein chains are folded up into complex shapes Examine three dimensional structure with X-ray diffraction Myoglobin first one examined All internal amino acids are nonpolar Hydrophobic interactions shove ...























