AP Chemistry Summer Assignment 2016
... Dimensional analysis is a way of problem solving that we will use to solve many (50% or more) of the problems this year. You must learn/review this process before the course begins or you will be lost for the remainder of this assignment and you will not understand much of problem solving techniques ...
... Dimensional analysis is a way of problem solving that we will use to solve many (50% or more) of the problems this year. You must learn/review this process before the course begins or you will be lost for the remainder of this assignment and you will not understand much of problem solving techniques ...
Organometallic Catalysts
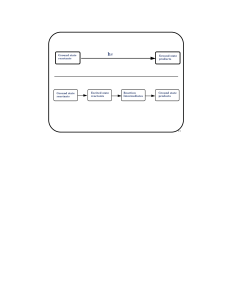
... The catalytically active species must have a vacant coordination site to allow the substrate to coordinate The establishment of a reaction mechanism is always a difficult task. It is even harder to definitively establish a catalytic cycle as all the reactions are going on in parallel! ...
... The catalytically active species must have a vacant coordination site to allow the substrate to coordinate The establishment of a reaction mechanism is always a difficult task. It is even harder to definitively establish a catalytic cycle as all the reactions are going on in parallel! ...
Chemistry 12 - hrsbstaff.ednet.ns.ca
... 2. A 10.0 g sample of a substance has 34.8 J of energy added to it and its temperature increases by 25.0°C. What is the specific heat capacity of the substance? A. 0.139 J/g°C B. 0.338 J/g°C C. 0.718 J/g°C D. 0.870 J/g°C 3. If the ΔH for a reaction is positive, which of the following statements is t ...
... 2. A 10.0 g sample of a substance has 34.8 J of energy added to it and its temperature increases by 25.0°C. What is the specific heat capacity of the substance? A. 0.139 J/g°C B. 0.338 J/g°C C. 0.718 J/g°C D. 0.870 J/g°C 3. If the ΔH for a reaction is positive, which of the following statements is t ...
Chemistry - Kendriya Vidyalaya Raigarh
... Bond Angle: It is defined as the angle between the orbitals containing bonding electron pairs around the central atom in a molecule/complex ion Bond Enthalpy: It is defined as the amount of energy required to break one mole of bonds of a particular type between two atoms in a gaseous state. Bond Ord ...
... Bond Angle: It is defined as the angle between the orbitals containing bonding electron pairs around the central atom in a molecule/complex ion Bond Enthalpy: It is defined as the amount of energy required to break one mole of bonds of a particular type between two atoms in a gaseous state. Bond Ord ...
Electrochemistry
... How to draw a Galvanic Cell The oxidation reaction occurs at the anode. The reduction reaction occurs at the cathode. You will be give the unbalanced net ionic reaction or a list of the substances present (line notation). From the information given you need to decide what half reactions occur in ea ...
... How to draw a Galvanic Cell The oxidation reaction occurs at the anode. The reduction reaction occurs at the cathode. You will be give the unbalanced net ionic reaction or a list of the substances present (line notation). From the information given you need to decide what half reactions occur in ea ...
Chemical Bonding II
... In ethylene, H2C=CH2, the sp2 orbitals are used to make one of the bonds between the carbon atoms and the bonds between carbon and hydrogen. These bonds have electron density along the internuclear (bond) axis. This type of bond is called a σ (sigma) bond. ...
... In ethylene, H2C=CH2, the sp2 orbitals are used to make one of the bonds between the carbon atoms and the bonds between carbon and hydrogen. These bonds have electron density along the internuclear (bond) axis. This type of bond is called a σ (sigma) bond. ...
Science Focus 9 Matter and Chemical Change Class Notes Topic 1
... In a chemical reaction, the total mass of the reactants, is always equal to the total mass of the products. This law ties in well with the atomic theory, which states that atoms are never created or destroyed. In a chemical reaction the atoms and molecules are simply rearranged. This law of conserva ...
... In a chemical reaction, the total mass of the reactants, is always equal to the total mass of the products. This law ties in well with the atomic theory, which states that atoms are never created or destroyed. In a chemical reaction the atoms and molecules are simply rearranged. This law of conserva ...
Unit 1 Notes
... 1. All matter is composed of extremely small particles called atoms, which cannot be broken into smaller particles, created nor destroyed. 2. The atoms of any given element are all identical to each other and different from the atoms of other elements. 3. Atoms of different elements combine in speci ...
... 1. All matter is composed of extremely small particles called atoms, which cannot be broken into smaller particles, created nor destroyed. 2. The atoms of any given element are all identical to each other and different from the atoms of other elements. 3. Atoms of different elements combine in speci ...
Principles in heterogeneous catalysis
... The contribution of this Teaching Unit to the development and command of the skills and learning outcomes of the programme(s) can be accessed at the end of this sheet, in the section entitled “Programmes/courses offering this Teaching Unit”. ...
... The contribution of this Teaching Unit to the development and command of the skills and learning outcomes of the programme(s) can be accessed at the end of this sheet, in the section entitled “Programmes/courses offering this Teaching Unit”. ...
Chemistry Notes for the Whole Year Powerpoint
... • Lewis structures are a 2-D representation of covalent molecules. • In order to make them, first split the molecule into its component elements. • Put Lewis dot symbols around each element. • Pair up unpaired electrons, on different atoms, to form covalent bonds (1 bond=2 shared electrons). • Put i ...
... • Lewis structures are a 2-D representation of covalent molecules. • In order to make them, first split the molecule into its component elements. • Put Lewis dot symbols around each element. • Pair up unpaired electrons, on different atoms, to form covalent bonds (1 bond=2 shared electrons). • Put i ...
Clathrates - An Exploration of the Chemistry of Caged Compounds
... Na gSi46, Nax Si 136 (X= 1-24) etc. are formed. Rb+ and Cs+ are too large to be incorporated into the cages of Si and Ge. Sn, however, forms clathrates with Rb + and Cs +yielding solids like RbzCS6 Sn46, Cs gSn46, K1.6CS6.4Sn44 and others. Figure 5 shows the inorganic clathrate structure. Inorganic ...
... Na gSi46, Nax Si 136 (X= 1-24) etc. are formed. Rb+ and Cs+ are too large to be incorporated into the cages of Si and Ge. Sn, however, forms clathrates with Rb + and Cs +yielding solids like RbzCS6 Sn46, Cs gSn46, K1.6CS6.4Sn44 and others. Figure 5 shows the inorganic clathrate structure. Inorganic ...
Reactions and Solutions - Louisiana Tech University
... reactions are subclassified as either single- or double-replacement. Types of Chemical Reactions Reactions that produce products with similar characteristics are often classified as a single group. For example, the formation of a precipitate denotes precipitation reactions. Chemical reactions that h ...
... reactions are subclassified as either single- or double-replacement. Types of Chemical Reactions Reactions that produce products with similar characteristics are often classified as a single group. For example, the formation of a precipitate denotes precipitation reactions. Chemical reactions that h ...
2.10 Alcohols notes - A
... becomes protonated by H+ ions which produces a water molecule which then leaves. The acid acts as a catalyst. The detailed mechanism is not required. The H which is lost comes from a carbon atom which is adjacent to the carbon atom attached to the OH group. In some cases, this can lead to more than ...
... becomes protonated by H+ ions which produces a water molecule which then leaves. The acid acts as a catalyst. The detailed mechanism is not required. The H which is lost comes from a carbon atom which is adjacent to the carbon atom attached to the OH group. In some cases, this can lead to more than ...
Document
... As explained above, condensation polymerisation is the process by which long-chain molecules are formed by reaction between bifunctional monomer molecules, with the loss of one small molecule (such as water or HCl) for each link which is formed. Normally there are two different monomer molecules, as ...
... As explained above, condensation polymerisation is the process by which long-chain molecules are formed by reaction between bifunctional monomer molecules, with the loss of one small molecule (such as water or HCl) for each link which is formed. Normally there are two different monomer molecules, as ...
The ontological autonomy of the chemical world - Philsci
... case to case, authors generally agree on considering that chemical concepts and descriptions cannot be derived from the concepts and the laws of physics as the epistemological reductionist supposes. For instance, Vemulapalli and Byerly (1999) claim that epistemological reduction fails even in relati ...
... case to case, authors generally agree on considering that chemical concepts and descriptions cannot be derived from the concepts and the laws of physics as the epistemological reductionist supposes. For instance, Vemulapalli and Byerly (1999) claim that epistemological reduction fails even in relati ...
Mill Hill County High School
... becomes protonated by H+ ions which produces a water molecule which then leaves. The acid acts as a catalyst. The detailed mechanism is not required. The H which is lost comes from a carbon atom which is adjacent to the carbon atom attached to the OH group. In some cases, this can lead to more than ...
... becomes protonated by H+ ions which produces a water molecule which then leaves. The acid acts as a catalyst. The detailed mechanism is not required. The H which is lost comes from a carbon atom which is adjacent to the carbon atom attached to the OH group. In some cases, this can lead to more than ...
THERMODYNAMICS OF REACTING SYSTEMS
... (moles of j present) = (moles of j originally present) + (moles of j produced by reaction) Moles of j produced by reaction are given by the product of the stoichiometric coefficient, ...
... (moles of j present) = (moles of j originally present) + (moles of j produced by reaction) Moles of j produced by reaction are given by the product of the stoichiometric coefficient, ...