
Substitution and Elimination Reactions . 7.1. Definitions.
... intermediate. Before we talk about how different compounds undergo substitution reactions at different rates, we need to discuss factors that affect the stability of carbocations. Let’s look at the electronic structure of a carbocation, for example the t-Bu cation. The central C atom is electron-def ...
... intermediate. Before we talk about how different compounds undergo substitution reactions at different rates, we need to discuss factors that affect the stability of carbocations. Let’s look at the electronic structure of a carbocation, for example the t-Bu cation. The central C atom is electron-def ...
3C95 Chemistry 12 2015-2016 (Lockwood)
... 1. explain why most reactions involve more than one step 2. describe a reaction mechanism as the series of steps (collisions) that result in the overall reaction and describe the role of the rate-determining step 3. explain the significance and role of a catalyst 4. identify reactant, product, react ...
... 1. explain why most reactions involve more than one step 2. describe a reaction mechanism as the series of steps (collisions) that result in the overall reaction and describe the role of the rate-determining step 3. explain the significance and role of a catalyst 4. identify reactant, product, react ...
Chemistry I
... Cl + e → Cl Negative ions = anions. anions. Cations derived from metal name (sodium (cat)ion (cat)ion,, silver (cat)ion (cat)ion)) or have the suffix –ium (NH4+ = ammonium ion) Anions from nonnon-metal atoms have the suffix –ide (chloride) in compounds with oxygen –ate (SO42-=sulfate) or –ite (SO32- ...
... Cl + e → Cl Negative ions = anions. anions. Cations derived from metal name (sodium (cat)ion (cat)ion,, silver (cat)ion (cat)ion)) or have the suffix –ium (NH4+ = ammonium ion) Anions from nonnon-metal atoms have the suffix –ide (chloride) in compounds with oxygen –ate (SO42-=sulfate) or –ite (SO32- ...
Solutions
... ‣ The quick story is molecules have a negative end and a positive end. ‣ The negative end of one molecule sticks to the positive end of another. ‣ We’ll discuss the rest in Chapter 11. ‣ Ionic Solids are held together by one type of intermolecular force. ‣ It’s a simpler story. ‣ The cations stick t ...
... ‣ The quick story is molecules have a negative end and a positive end. ‣ The negative end of one molecule sticks to the positive end of another. ‣ We’ll discuss the rest in Chapter 11. ‣ Ionic Solids are held together by one type of intermolecular force. ‣ It’s a simpler story. ‣ The cations stick t ...
Tables
... manner by a specialised waste contractor, or the local budget unit’s Hazardous Waste Safety Officer or the Work Environment Group contacted for advice on the treatment of the chemical. Peroxides may be removed using one of the two methods below: Method 1 Pass the solvent through a short column of ac ...
... manner by a specialised waste contractor, or the local budget unit’s Hazardous Waste Safety Officer or the Work Environment Group contacted for advice on the treatment of the chemical. Peroxides may be removed using one of the two methods below: Method 1 Pass the solvent through a short column of ac ...
Thermochemistry Thermodynamics is the study of energy and its
... Part 3. The heat of neutralization of HCl(aq) and NaOH(aq) Place 50.0 mL of 1.00 M HCl(aq) and the stir bar in the calorimeter, and 50.0 mL of 1.00 M NaOH(aq) in a graduated cylinder. Measure the temperature of the HCl and the NaOH with the thermometer. The two solutions should be at the same temper ...
... Part 3. The heat of neutralization of HCl(aq) and NaOH(aq) Place 50.0 mL of 1.00 M HCl(aq) and the stir bar in the calorimeter, and 50.0 mL of 1.00 M NaOH(aq) in a graduated cylinder. Measure the temperature of the HCl and the NaOH with the thermometer. The two solutions should be at the same temper ...
Chapter 11
... Stereochemistry, and possibility of rearrangements, depends on mechanism for each reaction ...
... Stereochemistry, and possibility of rearrangements, depends on mechanism for each reaction ...
1,1,1,5,5,5-hexafluoroacetylacetonato
... absorptivity coefficient of 7667 and 3889 L/mol cm respectively. This band at 287 nm is found in all β-diketones and their complexes for which upon chelation with a metal ion may be shifted either to longer or shorter wavelength with respect to the ligand. Barnum20 attributed these among others to t ...
... absorptivity coefficient of 7667 and 3889 L/mol cm respectively. This band at 287 nm is found in all β-diketones and their complexes for which upon chelation with a metal ion may be shifted either to longer or shorter wavelength with respect to the ligand. Barnum20 attributed these among others to t ...
Carbon and its Compounds Summary Study of the compounds of
... attaches itself to the dirt and grease. The short polar or ionic end of the soap molecule remains attached to water molecules. The latter form very small globules or structures called 'micelles' in which the oily dirt particle is surrounded with the tails of soap molecules carrying negative charge, ...
... attaches itself to the dirt and grease. The short polar or ionic end of the soap molecule remains attached to water molecules. The latter form very small globules or structures called 'micelles' in which the oily dirt particle is surrounded with the tails of soap molecules carrying negative charge, ...
Thermodynamics
... P (e.g. atmospheric pressure for a flask open to the air) and one at constant volume (for reactions in solution the volume changes are usually extremely small and so can be ignored) the enthalpy is essentially the energy of the system. (Pressure and volume changes are the main types of work performe ...
... P (e.g. atmospheric pressure for a flask open to the air) and one at constant volume (for reactions in solution the volume changes are usually extremely small and so can be ignored) the enthalpy is essentially the energy of the system. (Pressure and volume changes are the main types of work performe ...
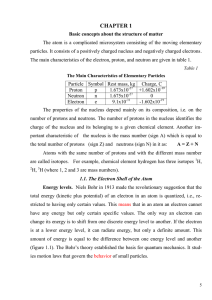
CHAPTER I
... Copper, in Group IB, will also have one electron assigned to the 4s orbital, plus 28 other electrons assigned to other orbitals. The configuration of Be 1s2 2s2.All elements of Group 2A have electron configurations [electrons of preceding rare gas + ns2], where n is the period in which the element ...
... Copper, in Group IB, will also have one electron assigned to the 4s orbital, plus 28 other electrons assigned to other orbitals. The configuration of Be 1s2 2s2.All elements of Group 2A have electron configurations [electrons of preceding rare gas + ns2], where n is the period in which the element ...
1.6 Energy changes in chemical reactions
... The traditional division of chemistry into physical, inorganic, and organic is an arbitrary one and the majority of chemists work across these divides. Most real problems also require chemists to interact with scientists in other disciplines. For example, chemists, physicists, mathematicians, and me ...
... The traditional division of chemistry into physical, inorganic, and organic is an arbitrary one and the majority of chemists work across these divides. Most real problems also require chemists to interact with scientists in other disciplines. For example, chemists, physicists, mathematicians, and me ...
Class XII Chemistry IMPORTANT QUESTIONS and COMMON
... Substances which are expected to possess paramagnetism or ferromagnetism on the basis of magnetic moments of the domains but actually possess zero net magnetic moment. e.g. MnO. It is due to the presence of equal number of domains in the opposite directions. Si doped with P is an n-type semi-conduct ...
... Substances which are expected to possess paramagnetism or ferromagnetism on the basis of magnetic moments of the domains but actually possess zero net magnetic moment. e.g. MnO. It is due to the presence of equal number of domains in the opposite directions. Si doped with P is an n-type semi-conduct ...
Student Review packet
... All problems involve stoichiometry: soluble salts, strong acids, strong bases Some problems involve equilibrium: “insoluble” salts, weak acids, weak bases For chemical reactions – Keq, Kc, and Kp are the important quantities For physical changes – Ka, Kb, Ksp, Kionize, and Kdissocation are the impor ...
... All problems involve stoichiometry: soluble salts, strong acids, strong bases Some problems involve equilibrium: “insoluble” salts, weak acids, weak bases For chemical reactions – Keq, Kc, and Kp are the important quantities For physical changes – Ka, Kb, Ksp, Kionize, and Kdissocation are the impor ...
Chemistry 11 – Course Review
... Element “X” is actually the real element ________________________________. Regions in space occupied by electrons are called ___________________________ Write the ground state electron configurations (eg. 1s2 2s2 2p6) for the following atoms or ions. You may use the core notation. a) ...
... Element “X” is actually the real element ________________________________. Regions in space occupied by electrons are called ___________________________ Write the ground state electron configurations (eg. 1s2 2s2 2p6) for the following atoms or ions. You may use the core notation. a) ...
Synthesis of [RuCl2(NO)2(THF)] and its Double CN BondForming
... investigation, methods for controlling the reactivity of nitric oxide at transition-metal centers have received considerably less attention.[1–3] For example, the migratory insertion of NO into metal–alkyl or metal–aryl bonds has been observed in only a handful of metal complexes,[3] despite the ana ...
... investigation, methods for controlling the reactivity of nitric oxide at transition-metal centers have received considerably less attention.[1–3] For example, the migratory insertion of NO into metal–alkyl or metal–aryl bonds has been observed in only a handful of metal complexes,[3] despite the ana ...
Dielectric and thermodynamic response of a
... functions provide a sensitive test of the effect of the long range interaction model on the dielectric response of the liquid. All simulations were performed in a cubic box containing 345 water molecules. In a few simulations, the box also contained one or two ions. The ion-water and ion-ion potenti ...
... functions provide a sensitive test of the effect of the long range interaction model on the dielectric response of the liquid. All simulations were performed in a cubic box containing 345 water molecules. In a few simulations, the box also contained one or two ions. The ion-water and ion-ion potenti ...
California Standards Practice - Student Edition
... 1. The periodic table displays the elements in increasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates to atomic structure. As a basis for understanding this concept: a. Students know how to relate the position of an element in the periodic ...
... 1. The periodic table displays the elements in increasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates to atomic structure. As a basis for understanding this concept: a. Students know how to relate the position of an element in the periodic ...




















![Synthesis of [RuCl2(NO)2(THF)] and its Double CN BondForming](http://s1.studyres.com/store/data/001773792_1-763ad0089529123821e01ed17077bbf2-300x300.png)

