* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chemical Bonding II
Rutherford backscattering spectrometry wikipedia , lookup
Homoaromaticity wikipedia , lookup
X-ray fluorescence wikipedia , lookup
2-Norbornyl cation wikipedia , lookup
Heat transfer physics wikipedia , lookup
Coupled cluster wikipedia , lookup
Hartree–Fock method wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Aromaticity wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Atomic theory wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Atomic orbital wikipedia , lookup
Chemical bond wikipedia , lookup
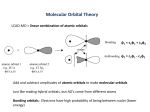
Chemical Bonding II Valence Bond & Molecular Orbital Theory Valence Bond Theory Orbital Overlap in Chemical Bonds Bonding Theories Hybridization The Valence Shell Electron Pair Repulsion approach works very well for many covalent compounds. It can be used to predict molecular shape, bond angles and molecular polarity. Several bonding theories have been developed to explain how the central atom rearranges its orbitals to minimize electronic repulsion. Bonding Theories Hybridization One of the simpler approaches involves hybridization, or the mixing of the atomic orbitals on the central atom. When orbitals hybridize, the properties of the resulting hybridized orbitals are a mixture of the properties of the original orbitals that were mixed together. Hybridization Bonding Theories Hybridization The compounds of carbon will be used for illustration. Methane, CH4, has four equivalent bonds which point towards the corners of a tetrahedron. Theorists have shown that the mixing of the 2s, 2px, 2py, and 2pz orbitals on carbon will produce four equivalent hybrid orbitals that are 109.5o apart. Bonding Theories Hybridization Since the hybrid orbitals result from mixing an s orbital with three p orbitals ( the 2px, 2py and 2pz ), the hybrid orbitals that result are called sp3 hybrid orbitals. Bonding Theories Hybridization When orbitals are mixed, a hybrid orbital is formed for each atomic orbital that is hybridized. In methane, a total of four atomic orbitals on carbon (2s, 2px, 2py and 2pz) produces four equivalent sp3 hybrid orbitals. Bonding Theories Hybridization Bonding Theories Hybridization Four equivalent bonds are formed with hydrogen to form a tetrahedral molecule. Hybridization of Methane Hybridization of Methane The hybridization of the orbitals on carbon accounts for the bond angles of the bonds in methane. However, atomic carbon has only two unpaired electrons, and would not be expected to make four bonds. ↓ 2p _ C: 1s2, 2s2, 2p2 =[He] 2s_ Hybridization of Methane Scientists proposed that an electron from the 2s level is promoted up to the 2p level. Hybridization of Methane The carbon atom can now make four bonds instead of two. Hybridization of Methane Hybridization of the 2s and 2p orbitals produces four equivalent hybrid orbitals. Hybridization of Methane Although the promotion of an electron and the mixing of orbitals requires energy, there is a net release of energy since four bonds are made, and repulsions are minimized. Sigma Bonds The bonds between carbon and hydrogen in methane puts electron density on the line connecting the nuclei of the atoms. This type of bond is called a σ (sigma) bond. All covalent molecules contain σ bonds. 3 sp Hybridization Molecules such as water or ammonia are also sp3 hybridized, with hybrid orbitals used for the bonds and any lone pairs of electrons. Hybridization Trigonal planar geometry results when an s orbital is mixed with two p orbitals. If the molecule is in the xy plane, the px and py orbitals are mixed with an s orbital to form three sp2 hybrid orbitals. The pz orbital remains unchanged, and can be used for making multiple bonds. Hybridization Hybridization Hybridization The energy level diagram shows the mixing of orbitals, with the pz orbital remaining unchanged. ↑ ↑ ↑ ↑ ↑ ↑ ↑ ↑ Hybridization The orbitals on the central atom show trigonal planar geometry, with the pz orbital perpendicular to the molecular plane. z xy plane Hybridization In ethylene, H2C=CH2, the sp2 orbitals are used to make one of the bonds between the carbon atoms and the bonds between carbon and hydrogen. These bonds have electron density along the internuclear (bond) axis. This type of bond is called a σ (sigma) bond. The σ Bonds of Ethylene Hybridization There are unhybridized pz orbitals on each of the carbon atoms, and they each contain an electron. A second bond forms between the carbon atoms. pz orbitals The Bond of Ethylene Bonds resulting from side-by-side overlap are called π (pi) bonds. The electrons in the π bond are found above and below the line connecting the two carbon nuclei. The Bond of Ethylene Even though there are two overlapping lobes (one above the molecular plane and one below it), this is only one π bond, as it involves the sharing of only one pair of electrons. σ and π Bonding Hybridization The Bonding of Ethylene sp Hybridization Molecules in which the central atom has two atoms attached to it (and no lone pairs of electrons) undergo sp hybridization. Acetylene, HCCH, has a triple bond between the carbon atoms. H-C=C-H sp Hybridization The shape of the molecule is linear around each carbon atom. The σ bonds between the carbons and between carbon and hydrogen result from the mixing of an s and p orbital on carbon. The result is two sp hybrid orbitals on each carbon. sp Hybridization If the molecule lies along the x axis, the px orbital on each carbon atom will be used in hybridization. This leaves the py and pz orbitals available for π bonding. sp Hybridization sp Hybridization z x x y sp Hybridization The σ bond between the carbon atoms arises from overlap of the sp orbitals. The bonds with the hydrogen atoms use the other sp hybrid orbital on each carbon atom. sp Hybridization There are two p orbitals (the py and pz) on each carbon atom. These are used to make two π bonds between the carbons. ↑ ↑ ↑ ↑ ↑ ↑ ↑ ↑ The Bonding in Acetylene The result is a σ bond along the C-C bond axis, and two π bonds which are perpendicular to each other. pz orbitals py orbitals The Bonding in Acetylene The result is a σ bond along the C-C bond axis, and two π bonds which are perpendicular to each other. sp Hybridization The end result is a carbon-carbon triple bond. H C C H The σ bonds are shown in yellow, and the π bonds are shown in red. 3 dsp Hybridization Central atoms with a total of 5 atoms and lone pairs form trigonal bipyramidal structures. 3 dsp Hybridization The central atom must be in the third period or below, since d orbitals are used to make five dsp3 or (sp3d) hybrid orbitals. 2 3 d sp Central atoms with a total of 6 atoms and electron pairs form octahedral shapes. Hybridization 2 3 d sp The hybridization is d2sp3 (or sp3d2), producing six hybrid orbitals that point toward the corners of an octahedron. Hybridization Molecular Orbital Theory Molecular Orbital Theory Valence Bond theory fails to fully explain the bonding in fairly simple molecules. These include molecules or ions with resonance. It also fails to fully explain the bonding of oxygen. Bonding of Oxygen Oxygen had a double bond which is predicted by the valence bond approach. : : : : O=O However, O2 is paramagnetic, and contains 2 unpaired electrons. The valence bond approach cannot explain the paramagnetism of oxygen. Molecular Orbital Theory Molecular orbital theory views bonds as resulting from the interaction of the wave functions on individual atoms. The waves can interact constructively or destructively. The resulting molecular orbitals belong to the entire molecule, and are not viewed as localized electron pairs. Molecular Orbital Theory The bonding orbital results in increased electron density between the two nuclei, and is of lower energy than the two separate atomic orbitals. Molecular Orbital Theory The antibonding orbital results in a node between the two nuclei, and is of greater energy than the two separate atomic orbitals. Molecular Orbital Theory Molecular Orbital Theory + - + + - + The signs on the molecular orbitals indicate the sign of the wave function, not ionic charge. Molecular Orbital Theory For hydrogen, the result is an energy level diagram with the bonding orbital occupied by a pair of electrons. The filling of the lower molecular orbital indicates that the molecule is stable compared to the two individual atoms. Rules for Combining Atomic Orbitals 1. 2. The number of molecular orbitals = the number of atomic orbitals combined. The strength of the bond depends upon the degree of orbital overlap. Orbital Overlap In order to overlap and form molecular orbitals, the atomic orbitals must have: 1. Proper symmetry to overlap 2. Comparable energies: The closer the two orbitals are in energy, the lower the energy of the bonding orbital, and the higher in energy the antibonding orbital. Period 2 Diatomic Molecules For the second period, assume that, due to a better energy match, s orbitals combine with s orbitals, and p orbitals combine with p orbitals. The symmetry of p orbitals permits end-onend overlap along the bond axis, or side-by-side overlap around, but not along, the internuclear axis. MOs using p orbitals + - - - + + - With the x axis as the bond axis, the px orbitals may combine constructively or destructively. The result is a σ bonding orbital and a σ anti-bonding orbital. MOs using p orbitals + - + - + - - The designation σ indicates symmetric electron density around the internuclear (x) axis. The + and – signs indicate the sign of the wave function, and not electrical charges. π Molecular Orbitals + - + + side-by-side overlap - p orbitals can also overlap side-by-side. Symmetry requires that the py orbital on one atom combines with the py orbital on the other atom. Similar overlap occurs between pz orbitals. π Molecular Orbitals + - + + side-by-side overlap - The orbital overlap side-by-side is less than that of overlap along the bond axis (end-on-end). As a result, the π bonding orbital will be higher in energy than the σ bond formed by the px orbitals. π Molecular Orbitals + - + + side-by-side overlap - π orbitals have electron density surrounding the bond axis, with a node along the internuclear axis. Molecular Orbital Diagram This is a molecular orbital energy level diagram for the p orbitals. Note that the σ bonding orbital is lowest in energy due to the greater overlap end-on-end. 2p 2p Molecular Orbital Diagrams 1. 2. 3. 4. Electrons preferentially occupy molecular orbitals that are lower in energy. Molecular orbitals may be empty, or contain one or two electrons. If two electrons occupy the same molecular orbital, they must be spin paired. When occupying degenerate molecular orbitals, electrons occupy separate orbitals with parallel spins before pairing. Molecular Orbital Diagrams Although molecular orbitals also result from the inner (core) electrons as well as valence electrons, many molecular orbital diagrams include only the valence level. Molecular Orbital Diagrams For O2, there will be a total of 12 valence electrons that must be placed in the diagram. Molecular Orbital Diagrams 2p 2s 2p 2s For O2, there will be a total of 12 valence electrons that must be placed in the diagram. Molecular Orbital Diagrams 2p 2s 2p 2s The molecular orbital diagram for oxygen shows two unpaired electrons, consistent with experimental data. Bond Order Bond order is an indicator of the bond strength and length. A bond order of 1 is equivalent to a single bond. Fractional bond orders are possible. The bond order of the molecule = (# e- in bonding orbtls) - (# e- in anti-bonding orbtls) 2 2 Molecular Orbital Diagrams 2p 2s 2p 2s The bond order of O2 is: 8-4 = 2 2 This is consistent with a double bond. Molecular Orbital Diagrams This energy level diagram works well for atoms in which the 2s and 2p levels are fairly far apart. These are the elements at the right of the table: O, F and Ne. MO diagram for Li through N The elements on the left side of period 2 have a fairly small energy gap between the 2s and 2p orbitals. As a result, interaction between s and p orbitals is possible. This can be viewed in different ways. MO diagram for Li through N In some approaches, the s orbital on one atom interacts with the p orbital on another. The interaction can be constructive or destructive. MO diagram for Li through N In another approach, the s and p orbitals on the same atom interact in what is called orbital mixing. Either approach yields the same result. The σ bonding and anti-bonding orbitals are raised in energy due to the interaction with a p orbital. MO diagram for Li through N MO diagram for N2 N2 has 10 valence electrons. Resonance The bonding in molecules with resonance aren’t accurately described using Lewis structures. An example is ozone, O3, which has two resonance structures: : : : : : : : : : : :O-O=O ↔ O=O-O: Resonance Either resonance structure provides the same molecular shape- bent with an angle of approximately 120o. However, both bonds are identical in length and strength. The bonds are intermediate between single bonds and double bonds. Resonance Resonance A more accurate description of the bonding can be obtained by considering the π bonding to be delocalized, or spread out over all three oxygen atoms. Valence Bond & Molecular Orbital Theory The delocalized or extended pi system is consistent with the lowest energy bonding orbitals obtained using molecular orbital theory. Extended Bonding Extended Bonding Extended Bonding Extended π Bonding – Nitrate Bonding in Metals A simple model of the bonding of metals is called the electron sea model. The structure if viewed as metal ions sitting in a sea of valence electrons. Since the electrons are not associated with a particular nucleus or atom, they are free to move and conduct an electrical current. Bonding in Metals Metals are malleable (can be pounded into thin sheets) and ductile (can be drawn into wires). These properties suggest that there are no localized bonds between the metal atoms, so the structure can be easily deformed without breaking. Band Theory A more sophisiticated description of the bonding in metallic solids is called band theory. This approach applies molecular orbital theory (the constructive and destructive interaction of atomic orbitals) to create orbitals that are delocalized over the entire solid crystal. Band Theory The greater number of atoms in the crystal results in molecular orbitals that are very close in energy, resulting in a continuous band of energies. Band Theory Semiconductors In a conductor, there is no energy gap between the valence band and the conduction band. In a semiconductor, there is a small energy gap, and electrons can be promoted into the conduction band. In insulators, the gap is too large for electrons to enter the conduction band. Semiconductors