19 BROWN Chemical Thermodynamics PPTSExercise
... Plan: In part (a) we can make this prediction by determining the sign of ΔS° for the reaction and then using that information to analyze Equation 19.12. In part (b) we need to calculate ΔH° and ΔS° for the reaction by using the data in Appendix C. We can then use Equation 19.12 to calculate ΔG°. Sol ...
... Plan: In part (a) we can make this prediction by determining the sign of ΔS° for the reaction and then using that information to analyze Equation 19.12. In part (b) we need to calculate ΔH° and ΔS° for the reaction by using the data in Appendix C. We can then use Equation 19.12 to calculate ΔG°. Sol ...
Slide 1
... Plan: In part (a) we can make this prediction by determining the sign of ΔS° for the reaction and then using that information to analyze Equation 19.12. In part (b) we need to calculate ΔH° and ΔS° for the reaction by using the data in Appendix C. We can then use Equation 19.12 to calculate ΔG°. Sol ...
... Plan: In part (a) we can make this prediction by determining the sign of ΔS° for the reaction and then using that information to analyze Equation 19.12. In part (b) we need to calculate ΔH° and ΔS° for the reaction by using the data in Appendix C. We can then use Equation 19.12 to calculate ΔG°. Sol ...
CHAPTER-7 EQUILIBRIUM Equilibrium state- When
... Buffer solution :The solutions which resist change in pH on dilution or with the addition of small amounts of acid or alkali are called Buffer Solutions. common ion effect: It can be defined as a shift in equilibrium on adding a substance that provides more of an ionic species already present in ...
... Buffer solution :The solutions which resist change in pH on dilution or with the addition of small amounts of acid or alkali are called Buffer Solutions. common ion effect: It can be defined as a shift in equilibrium on adding a substance that provides more of an ionic species already present in ...
Topic 3: Chemical Kinetics - Manitoba Education and Training
... Demonstrate appropriate scientific skills when seeking answers to questions. Demonstrate curiosity, skepticism, creativity, open-mindedness, accuracy, precision, honesty, and persistence, and appreciate their importance as scientific and technological habits of mind. Understand the properties and st ...
... Demonstrate appropriate scientific skills when seeking answers to questions. Demonstrate curiosity, skepticism, creativity, open-mindedness, accuracy, precision, honesty, and persistence, and appreciate their importance as scientific and technological habits of mind. Understand the properties and st ...
am 06 chemistry - University of Malta
... (i) Give a cell diagram for the reaction using the usual cell notation. (ii) In which direction will electrons flow in the external circuit? Justify your answer. (iii) Describe what, if anything, will happen to the colour intensity of the solution of the Br2(aq)/Br– (aq) half cell. (iv) Write a bala ...
... (i) Give a cell diagram for the reaction using the usual cell notation. (ii) In which direction will electrons flow in the external circuit? Justify your answer. (iii) Describe what, if anything, will happen to the colour intensity of the solution of the Br2(aq)/Br– (aq) half cell. (iv) Write a bala ...
380 KB / 39 pages
... chemical species. (d) When a glass of ice cubes and water is left overnight and there are no ice cubes in the water the next morning, no chemical reaction has occurred. The ice cubes melted, H2O(s) → H2O(l), but no new chemical species formed. Problem 6.2. (a) When a match is dropped on the floor, n ...
... chemical species. (d) When a glass of ice cubes and water is left overnight and there are no ice cubes in the water the next morning, no chemical reaction has occurred. The ice cubes melted, H2O(s) → H2O(l), but no new chemical species formed. Problem 6.2. (a) When a match is dropped on the floor, n ...
MECH 558 Combustion Class Notes
... Before learning how hydrocarbons react with oxygen in flames, we must first go over some nomenclature for the different classes of hydrocarbons. 2.1. Alkanes (paraffins): These molecules consist of carbon atoms which are all connected by single bonds and are saturated with hydrogen atoms (i.e. no mo ...
... Before learning how hydrocarbons react with oxygen in flames, we must first go over some nomenclature for the different classes of hydrocarbons. 2.1. Alkanes (paraffins): These molecules consist of carbon atoms which are all connected by single bonds and are saturated with hydrogen atoms (i.e. no mo ...
Physical Chemistry 3: — Chemical Kinetics
... The scriptum gives a summary of the material covered in the scheduled lectures to allow students to repeat the material more economically. It covers basic material that all chemistry students should learn irrespective of their possible inclination towards inorganic, organic or physical chemistry, bu ...
... The scriptum gives a summary of the material covered in the scheduled lectures to allow students to repeat the material more economically. It covers basic material that all chemistry students should learn irrespective of their possible inclination towards inorganic, organic or physical chemistry, bu ...
FUNCTIONAL GROUP IDENTIFICATION WORKSHEET
... Be able to identify and name alcohols. Be able to identify and name ethers. ...
... Be able to identify and name alcohols. Be able to identify and name ethers. ...
REACTION PREDICTION
... Double Replacement (metathesis) Two compounds react to form two new compounds. All double replacement reactions must have a "driving force" that removes a pair of ions from solution. Ions keep their same charges as reactants and products. Formation of a precipitate: A precipitate is an insoluble su ...
... Double Replacement (metathesis) Two compounds react to form two new compounds. All double replacement reactions must have a "driving force" that removes a pair of ions from solution. Ions keep their same charges as reactants and products. Formation of a precipitate: A precipitate is an insoluble su ...
Deans Community High School Intermediate 2 Revision Notes www
... Uses of catalyst. As we have seen, reactions are more likely to take place when high concentrations, large surface areas and high temperatures are used. These factors increase the likelihood of collisions of the reactants, and the more energy that these collision have, the more likely it will be tha ...
... Uses of catalyst. As we have seen, reactions are more likely to take place when high concentrations, large surface areas and high temperatures are used. These factors increase the likelihood of collisions of the reactants, and the more energy that these collision have, the more likely it will be tha ...
It`s Easy Being a Green Chemist
... Unnecessary derivatization (use of blocking groups, protection/ deprotection, temporary modification of physical/chemical processes) should be minimized or avoided if possible, because such steps require additional reagents and can generate waste. 9) Catalysis Catalytic reagents (as selective as pos ...
... Unnecessary derivatization (use of blocking groups, protection/ deprotection, temporary modification of physical/chemical processes) should be minimized or avoided if possible, because such steps require additional reagents and can generate waste. 9) Catalysis Catalytic reagents (as selective as pos ...
Metal disordering Cu(II) supramolecular polymers constructed from
... Remarkably, whatever arrangements the copper atoms are assumed, the stacking of the whole network results in two different kinds of hydrophilic channels running parallel to the c axis (Fig. 2d). The hexagonal ones are composed of the copper centers and the bridging carboxylate groups with the effect ...
... Remarkably, whatever arrangements the copper atoms are assumed, the stacking of the whole network results in two different kinds of hydrophilic channels running parallel to the c axis (Fig. 2d). The hexagonal ones are composed of the copper centers and the bridging carboxylate groups with the effect ...
Advanced Placement Chemistry Test
... equal to, or less than the initial concentration of HI(g). Justify your answer. {3 marks} ...
... equal to, or less than the initial concentration of HI(g). Justify your answer. {3 marks} ...
The Solubility of Potassium Sulfate in Thermodynamic view
... interactions between them are negligible. Under these circumstance the ions behavior is independent from each other and the electrolyte behaves almost as an ideal solution. As the concentration increases, the average distance between the ions decreases, so interactions between them become considerab ...
... interactions between them are negligible. Under these circumstance the ions behavior is independent from each other and the electrolyte behaves almost as an ideal solution. As the concentration increases, the average distance between the ions decreases, so interactions between them become considerab ...
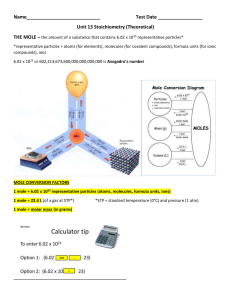
Unit 13 Stoichiometry (Theoretical)
... b. How many grams of acetylene (C2H2) are produced by adding water to 5.00 grams of CaC2? (ans. 2.03 g C2H2) ...
... b. How many grams of acetylene (C2H2) are produced by adding water to 5.00 grams of CaC2? (ans. 2.03 g C2H2) ...
ALDEHYDES AND KETONES I. NUCLEOPHILIC ADDITION TO …
... a chemical reaction into another known compound -- a derivative • If the melting point of the derivative matches the expected value, according to the literature, then one can assume that the ...
... a chemical reaction into another known compound -- a derivative • If the melting point of the derivative matches the expected value, according to the literature, then one can assume that the ...
unit 17 organic compounds containing oxygen and nitrogen atoms
... ketons with general formula RCOR'. Aldehydes and ketones have the same general formula CnH,"O. Since carbonyl group is present in both aldehydes and ketones, many of their properties are common. But in aldehyde, there is also a reactive hydrogen atom, which confers reducing properties upon the molec ...
... ketons with general formula RCOR'. Aldehydes and ketones have the same general formula CnH,"O. Since carbonyl group is present in both aldehydes and ketones, many of their properties are common. But in aldehyde, there is also a reactive hydrogen atom, which confers reducing properties upon the molec ...
Design and Construction of Coordination Polymers Brochure
... chemistry and materials. Design and Construction of Coordination Polymers provides a comprehensive introduction to this field, focusing on synthetic strategies, structures, properties, and potential applications. Each chapter provides a unique perspective on coordination polymers, offering a dedicat ...
... chemistry and materials. Design and Construction of Coordination Polymers provides a comprehensive introduction to this field, focusing on synthetic strategies, structures, properties, and potential applications. Each chapter provides a unique perspective on coordination polymers, offering a dedicat ...
Ex. 32 Answer
... Paracetamol is a relatively safe drug but toxic side effects have been observed with high doses greater than 10 – 15 g. The principal toxic metabolite of paracetamol is N-acetyl-p-benzoquinone imine (NAPQI). The tripeptide glutathione stored in the liver can combine with this metabolite, producing a ...
... Paracetamol is a relatively safe drug but toxic side effects have been observed with high doses greater than 10 – 15 g. The principal toxic metabolite of paracetamol is N-acetyl-p-benzoquinone imine (NAPQI). The tripeptide glutathione stored in the liver can combine with this metabolite, producing a ...