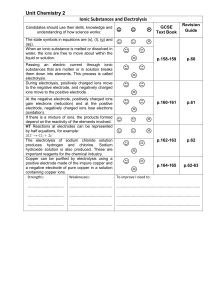
Molecules, Compounds, and Chemical Equations (Chapter 3)
... Adjust coefficients to get equal numbers of each kind of element on both sides of arrow. Use smallest, whole number coefficients. e.g., start with unbalanced equation (for the combustion of butane): C4H10 + O2 reactants ...
... Adjust coefficients to get equal numbers of each kind of element on both sides of arrow. Use smallest, whole number coefficients. e.g., start with unbalanced equation (for the combustion of butane): C4H10 + O2 reactants ...
Name___________________________________________ Date_________________________ Algebra I – Pd ____ Complex Equations
... Name___________________________________________ Date_________________________ Algebra I – Pd ____ Complex Equations 2A ...
... Name___________________________________________ Date_________________________ Algebra I – Pd ____ Complex Equations 2A ...
Review Sheet
... affects the number of each atom in that formula. c. Sometimes an element is found in more than one compound on the same side of an equation, which can make it even more challenging to balance. d. There are times when you may have to change a coefficient more than once before the entire equation is b ...
... affects the number of each atom in that formula. c. Sometimes an element is found in more than one compound on the same side of an equation, which can make it even more challenging to balance. d. There are times when you may have to change a coefficient more than once before the entire equation is b ...
Math 285: Differential Equations Quiz 6: Solutions 1. Verify that the
... We look for a second solution of the form y = ux. We have y 0 = u0 x + u y 00 = u00 x + 2u0 . Substituting into the differential equation we obtain: x2 (u00 x + 2u0 ) + x(u0 x + u) − ux = x2 (u00 x + 3u0 ) = 0. Dividing through by x2 and substituting w = u0 we obtain the first order homogeneous line ...
... We look for a second solution of the form y = ux. We have y 0 = u0 x + u y 00 = u00 x + 2u0 . Substituting into the differential equation we obtain: x2 (u00 x + 2u0 ) + x(u0 x + u) − ux = x2 (u00 x + 3u0 ) = 0. Dividing through by x2 and substituting w = u0 we obtain the first order homogeneous line ...
General Chemistry I
... The element magnesium is found in nature as three isotopes with masses and abundances as follows: 24Mg: 23.9924 amu, 78.70%; 25 Mg: 24.9938 amu, 10.13% and 26Mg: 25.9898 amu, 11.17%. Calculate the average atomic weight magnesium. ...
... The element magnesium is found in nature as three isotopes with masses and abundances as follows: 24Mg: 23.9924 amu, 78.70%; 25 Mg: 24.9938 amu, 10.13% and 26Mg: 25.9898 amu, 11.17%. Calculate the average atomic weight magnesium. ...
Classifying Chemical Reactions 9-3
... A chemical change is really a chemical reaction Has two parts: • Reactants: the substances you start with • Products: the substances you end up with ...
... A chemical change is really a chemical reaction Has two parts: • Reactants: the substances you start with • Products: the substances you end up with ...
Balancing ANY chemical Equation
... What is a weak electrolyte? • Electrolytes: Substances that form ions when dissolved in solution. Electrolytes can be weak or strong. • Strong Electrolytes: Substances that completely separate into their component ions when dissolved. (All soluble ionic compounds and strong acids are strong electro ...
... What is a weak electrolyte? • Electrolytes: Substances that form ions when dissolved in solution. Electrolytes can be weak or strong. • Strong Electrolytes: Substances that completely separate into their component ions when dissolved. (All soluble ionic compounds and strong acids are strong electro ...
Review Packet
... Given the following reactants predict the products. Add if the reactions are classified as Acid-Base in addition to the other classifications. ...
... Given the following reactants predict the products. Add if the reactions are classified as Acid-Base in addition to the other classifications. ...
Packet
... Given the following reactants predict the products. Add if the reactions are classified as Acid-Base in addition to the other classifications. ...
... Given the following reactants predict the products. Add if the reactions are classified as Acid-Base in addition to the other classifications. ...