PowerPoint 1
... A linear equation in one variable is an equation that can be written in the form ax = b where a and b are constants and a 0. A number is a solution of an equation if the statement is true when the number is substituted for the variable. Two equations are equivalent if they have the same solutions. ...
... A linear equation in one variable is an equation that can be written in the form ax = b where a and b are constants and a 0. A number is a solution of an equation if the statement is true when the number is substituted for the variable. Two equations are equivalent if they have the same solutions. ...
Unit 3 Review Notes - Brinkmann chapter7_and_8_review1
... • Arrange atoms - singular atom is usually in the middle. • Form bonds between atoms (2 e-). • Distribute remaining e- to give each atom an octet (recall exceptions). • If there aren’t enough e- to go around, form double or triple bonds. ...
... • Arrange atoms - singular atom is usually in the middle. • Form bonds between atoms (2 e-). • Distribute remaining e- to give each atom an octet (recall exceptions). • If there aren’t enough e- to go around, form double or triple bonds. ...
Writing Formulas
... Binary compounds consist of two elements that may be ionic or covalent. The general rule is to put the least electronegative element first, the more electronegative element second and balance the charges to zero. ...
... Binary compounds consist of two elements that may be ionic or covalent. The general rule is to put the least electronegative element first, the more electronegative element second and balance the charges to zero. ...
PED-HSM11A2TR-08-1103-001
... Beth earns $25 per week. Total earned: $275 an equation to find the number of weeks it takes to earn $275 together ...
... Beth earns $25 per week. Total earned: $275 an equation to find the number of weeks it takes to earn $275 together ...
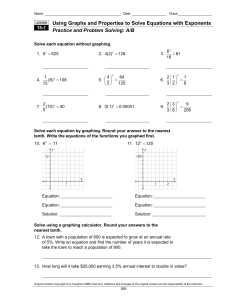
Using Graphs and Properties to Solve Equations with Exponents
... of 5%. Write an equation and find the number of years it is expected to take the town to reach a population of 900. _________________________________________________________________________________________ ...
... of 5%. Write an equation and find the number of years it is expected to take the town to reach a population of 900. _________________________________________________________________________________________ ...
Empirical Formula Lab
... So what are we trying to do? By figuring out how much zinc and chlorine are in our product (zinc chloride), we hope to determine the molar ratio between the two elements Things we need….. • Mass of Zn in product -we will assume ALL of the zinc we had at the beginning successfully reacted so the amo ...
... So what are we trying to do? By figuring out how much zinc and chlorine are in our product (zinc chloride), we hope to determine the molar ratio between the two elements Things we need….. • Mass of Zn in product -we will assume ALL of the zinc we had at the beginning successfully reacted so the amo ...
Molecules and Ions
... Empirical Formula: the lowest whole number ratio of each type of atom in a compound e.g. hydrogen peroxide = HO ...
... Empirical Formula: the lowest whole number ratio of each type of atom in a compound e.g. hydrogen peroxide = HO ...
Ch. 9
... – F-(fluoride), Cl-(chloride), O2-(oxide), S2-(sulfide), N3(nitride), P3-(phosphide), As3- (arsenide) ...
... – F-(fluoride), Cl-(chloride), O2-(oxide), S2-(sulfide), N3(nitride), P3-(phosphide), As3- (arsenide) ...
Molecules and Ions
... Empirical Formula: the lowest whole number ratio of each type of atom in a compound e.g. hydrogen peroxide = HO ...
... Empirical Formula: the lowest whole number ratio of each type of atom in a compound e.g. hydrogen peroxide = HO ...
The Slope-Intercept Formula
... These are special equations. When you only see one variable, that means the line only touches one axis. ...
... These are special equations. When you only see one variable, that means the line only touches one axis. ...
Name: Date: AP Chemistry/Chemistry 145 Summer Assignment
... 19. A 11.6-g sample of iron ore, containing Fe3O4 (232 g/mol), is reacted with carbon to form purified iron. The other product of the reaction is carbon dioxide gas. 2.10 g of iron is ...
... 19. A 11.6-g sample of iron ore, containing Fe3O4 (232 g/mol), is reacted with carbon to form purified iron. The other product of the reaction is carbon dioxide gas. 2.10 g of iron is ...
Ionic Compounds 1. What is the formula for aluminum phosphate
... 2. A 87.2-g sample of SrCl2 is dissolved in 112.5 mL of solution. Calculate the molarity of this solution. 3. How many grams of NaCl are contained in 350. mL of a 0.171 M solution of sodium chloride? 4. What mass of calcium chloride, CaCl2, is in 3.576 L of a 1.56 M solution? 5. Which of the followi ...
... 2. A 87.2-g sample of SrCl2 is dissolved in 112.5 mL of solution. Calculate the molarity of this solution. 3. How many grams of NaCl are contained in 350. mL of a 0.171 M solution of sodium chloride? 4. What mass of calcium chloride, CaCl2, is in 3.576 L of a 1.56 M solution? 5. Which of the followi ...
Math 285: Differential Equations Quiz 7: Solutions 1. Solve the given
... y 00 − 2y 0 − 3y = 6 − 2e−x First we consider the associated homogeneous equation. The auxiliary equation is m2 − 2m − 3 = 0. The roots are m = 3, −1 and therefore the complementary solution is yc = c1 e3x + c2 e−x . To find a particular solution to the nonhomogeneous equation using the method of un ...
... y 00 − 2y 0 − 3y = 6 − 2e−x First we consider the associated homogeneous equation. The auxiliary equation is m2 − 2m − 3 = 0. The roots are m = 3, −1 and therefore the complementary solution is yc = c1 e3x + c2 e−x . To find a particular solution to the nonhomogeneous equation using the method of un ...
formula mass.
... of any gas under the same conditions has the same number of molecules. Johann Josef Loschmidt, a German physicist, named and discovered the Avogadro number. Loschmidt realized that a mole of any substance—be it a gas, liquid, or solid— contains 6.02 x 1023 atoms or molecules. ...
... of any gas under the same conditions has the same number of molecules. Johann Josef Loschmidt, a German physicist, named and discovered the Avogadro number. Loschmidt realized that a mole of any substance—be it a gas, liquid, or solid— contains 6.02 x 1023 atoms or molecules. ...