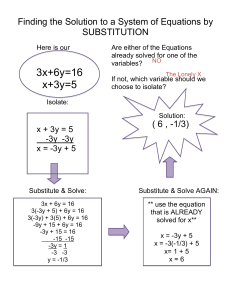
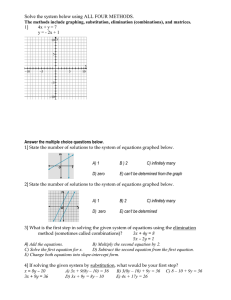
gr11chemreview
... 20. A sample of copper (II) sulphate pentahydrate weighs 6.8 g. What mass of the sample is due to the water? ...
... 20. A sample of copper (II) sulphate pentahydrate weighs 6.8 g. What mass of the sample is due to the water? ...
First Order Linear Differential Equations
... dx dy M ( x) M ( x)3 y M ( x) x dx p ( x ) dx M ( x) e M ( x ) e 3 x dy e 3 x 3e 3 x y xe3 x dx d 3 x (e . y ) xe3 x dx xe3 x e 3 x 3 x e .y ...
... dx dy M ( x) M ( x)3 y M ( x) x dx p ( x ) dx M ( x) e M ( x ) e 3 x dy e 3 x 3e 3 x y xe3 x dx d 3 x (e . y ) xe3 x dx xe3 x e 3 x 3 x e .y ...
Topics on Chapter 10 Test: The Mole
... material that cause allergic response, such as pollen. Histamine causes dilation of blood vessels and swelling due to accumulation of fluid in the tissues. People sometimes take antihistamine drugs to counteract the effects of histamine. A sample of histamine having a mass of 385 mg is composed of 2 ...
... material that cause allergic response, such as pollen. Histamine causes dilation of blood vessels and swelling due to accumulation of fluid in the tissues. People sometimes take antihistamine drugs to counteract the effects of histamine. A sample of histamine having a mass of 385 mg is composed of 2 ...
C - Upton-by-Chester High School
... A hydrocarbon was found to contain 82.8% by mass of carbon. It has an Mr of 58. Find the empirical (see working below) C2H5 and molecular formulas of this compound. The empirical formula has a mass of 29. 58/29 = 2, so we need double the molecular formula C4H10 C ...
... A hydrocarbon was found to contain 82.8% by mass of carbon. It has an Mr of 58. Find the empirical (see working below) C2H5 and molecular formulas of this compound. The empirical formula has a mass of 29. 58/29 = 2, so we need double the molecular formula C4H10 C ...
Study Guide for Exam 2_old
... You should be able to answer the following questions, solve problems involving the following concepts, or understand the following concepts so that you can describe them and answer questions about them. Periodic trends regarding atomic and ionic radii. What is meant by valence electrons? What is ion ...
... You should be able to answer the following questions, solve problems involving the following concepts, or understand the following concepts so that you can describe them and answer questions about them. Periodic trends regarding atomic and ionic radii. What is meant by valence electrons? What is ion ...
Unit 9 – Behavior of Gases
... 22. Calculate the volume of a gas at a pressure of 100. kPa if its volume at 102 kPa is 1.50 x 103 mL. 23. The gas in a closed container has a pressure of 300. kPa at 30oC (303K). What will the pressure be if the temperature is lowered to –127oC? 24. A sealed cylinder of gas contains nitrogen gas at ...
... 22. Calculate the volume of a gas at a pressure of 100. kPa if its volume at 102 kPa is 1.50 x 103 mL. 23. The gas in a closed container has a pressure of 300. kPa at 30oC (303K). What will the pressure be if the temperature is lowered to –127oC? 24. A sealed cylinder of gas contains nitrogen gas at ...
Stoichiometry 1 amu = 1.6606 x 10-24 g The amu mass of an atom
... Stoichiometry 1 amu = 1.6606 x 10-24 g The amu mass of an atom of carbon-12 is 12 amu 1 mole = count multiplier = 6.022 x 1023 items subscript to the right of an element symbol = atom count multiplier = the number of atoms of the element in a chemical formula number before chemical formula in a chem ...
... Stoichiometry 1 amu = 1.6606 x 10-24 g The amu mass of an atom of carbon-12 is 12 amu 1 mole = count multiplier = 6.022 x 1023 items subscript to the right of an element symbol = atom count multiplier = the number of atoms of the element in a chemical formula number before chemical formula in a chem ...
Bonding Notes
... non-metals does demonstrate one method to arrive at a viable formula for an ionic compound. Each atom must either lose (metals) or gain (non-metals) enough electrons to fill or empty its valence shell. This determines the formula, because an particular number of atoms of each element are needed to a ...
... non-metals does demonstrate one method to arrive at a viable formula for an ionic compound. Each atom must either lose (metals) or gain (non-metals) enough electrons to fill or empty its valence shell. This determines the formula, because an particular number of atoms of each element are needed to a ...
The concentration of OH- ions in a certain household ammonia
... The pH scale is based on the auto-ionization of water. At 25oC, water ionizes to a small extent according to the below equation: H2O(l) H+(aq) + OH-(aq) An equilibrium expression can be written for the above equation, which is: Kw = [H+][OH-] = 1.00 x 10-14 Notice that water doesn’t appear in the ...
... The pH scale is based on the auto-ionization of water. At 25oC, water ionizes to a small extent according to the below equation: H2O(l) H+(aq) + OH-(aq) An equilibrium expression can be written for the above equation, which is: Kw = [H+][OH-] = 1.00 x 10-14 Notice that water doesn’t appear in the ...
Ch. 3 - Chemical Reactions
... Describing Equations Zn(s) + 2HCl(aq) ZnCl2(aq) + H2(g) • How many? • Of what? • In what state? ...
... Describing Equations Zn(s) + 2HCl(aq) ZnCl2(aq) + H2(g) • How many? • Of what? • In what state? ...
Chemical Reactions and Reaction Stoichiometry
... When a hydrocarbon is fully combusted, the mass of water and carbon dioxide collected can be used directly to determine the amount of carbon and hydrogen in the original compound. ...
... When a hydrocarbon is fully combusted, the mass of water and carbon dioxide collected can be used directly to determine the amount of carbon and hydrogen in the original compound. ...
CHM134 General Chemistry I Semester Review – Dr. Steel This list
... CHM134 General Chemistry I Semester Review – Dr. Steel This list represents the most important topics we covered in CHM134 this semester. It is not a complete list of every topic that might appear on the final exam. Formulas and Constants K = °C + 273 M D= 1 in = 2.54 cm V metric conversions ...
... CHM134 General Chemistry I Semester Review – Dr. Steel This list represents the most important topics we covered in CHM134 this semester. It is not a complete list of every topic that might appear on the final exam. Formulas and Constants K = °C + 273 M D= 1 in = 2.54 cm V metric conversions ...
Chapter 8
... matter can neither be created nor destroyed, but it can change forms chemical equations must show that matter was conserved ...
... matter can neither be created nor destroyed, but it can change forms chemical equations must show that matter was conserved ...