Study Guide for Exam 2_Sp12
... Periodic trends regarding atomic and ionic radii. What is meant by valence electrons? What is ionization energy? What is the octet rule? What are ions? How do they relate to the octet rule? How is charge balance related to writing formulas of ionic compounds? Write dot formulas for atoms, and show h ...
... Periodic trends regarding atomic and ionic radii. What is meant by valence electrons? What is ionization energy? What is the octet rule? What are ions? How do they relate to the octet rule? How is charge balance related to writing formulas of ionic compounds? Write dot formulas for atoms, and show h ...
Literal Equations and Formulas
... – You can "rewrite" a literal equation to isolate any one of the variables using inverse operations. This is called solving for a variable. – When you rewrite literal equations, you may have to divide by a variable or variable expression. In this lesson, assume that the variable or variable expressi ...
... – You can "rewrite" a literal equation to isolate any one of the variables using inverse operations. This is called solving for a variable. – When you rewrite literal equations, you may have to divide by a variable or variable expression. In this lesson, assume that the variable or variable expressi ...
Ionic vs Molecular Compounds Name Period Unit 4 – HW 1
... 16. Chemical formulas can also be written for ionic compounds. In this case, however, the formula does ...
... 16. Chemical formulas can also be written for ionic compounds. In this case, however, the formula does ...
Review1
... 2. A spring–mass system has a spring constant of 3 N / m . A mass of 2 kg is attached to the spring and the motion takes place in a viscous fluid that offers a resistance numerically equal to the magnitude of the instantaneous velocity. If the system is driven by an external force of (3cos 3t - 2sin ...
... 2. A spring–mass system has a spring constant of 3 N / m . A mass of 2 kg is attached to the spring and the motion takes place in a viscous fluid that offers a resistance numerically equal to the magnitude of the instantaneous velocity. If the system is driven by an external force of (3cos 3t - 2sin ...
Section 1.2
... pays time-and-a-half for all hours worked in excess of 40 hours. Determine Ann’s regular hourly wage. ...
... pays time-and-a-half for all hours worked in excess of 40 hours. Determine Ann’s regular hourly wage. ...
fourth and final quiz
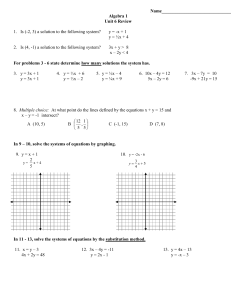
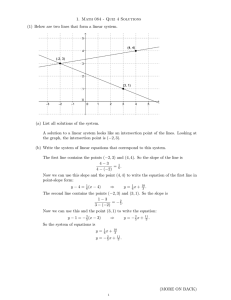
... (a) List all solutions of the system. A solution to a linear system looks like an intersection point of the lines. Looking at the graph, the intersection point is (−2, 3). (b) Write the system of linear equations that correspond to this system. The first line contains the points (−2, 3) and (4, 4). ...
... (a) List all solutions of the system. A solution to a linear system looks like an intersection point of the lines. Looking at the graph, the intersection point is (−2, 3). (b) Write the system of linear equations that correspond to this system. The first line contains the points (−2, 3) and (4, 4). ...
Review Sheet for Unit 4 Test
... 1. How many carbon atoms are in 125.0 g of glucose (C6H12O6)? 2. What is the mass of one million (1.00 x 106) gold atoms? 3. 3.76 x 1022 atoms of a metal have a mass of 7.020 g. What is the metal? 4. Butanoic acid is composed of 54.53% carbon, 36.32% oxygen, and 9.15% hydrogen. What is its empirical ...
... 1. How many carbon atoms are in 125.0 g of glucose (C6H12O6)? 2. What is the mass of one million (1.00 x 106) gold atoms? 3. 3.76 x 1022 atoms of a metal have a mass of 7.020 g. What is the metal? 4. Butanoic acid is composed of 54.53% carbon, 36.32% oxygen, and 9.15% hydrogen. What is its empirical ...
1 - KCSE Online
... (d) G has a larger atomic radius than C hence √1mk the metallic bonds in C is stronger than in G √1mk T.T 2mks (e) Giant atomic structure / molecular structre√1mk (f) P has a smaller atomic radius than O due to a larger nuclear charge which pulls electrons strongly √1mk since added electrons enter t ...
... (d) G has a larger atomic radius than C hence √1mk the metallic bonds in C is stronger than in G √1mk T.T 2mks (e) Giant atomic structure / molecular structre√1mk (f) P has a smaller atomic radius than O due to a larger nuclear charge which pulls electrons strongly √1mk since added electrons enter t ...