CHEMICAL EQUATIONS NAME PERIOD_______ DATE________
... A ______________ is a well-defined example of a chemical change. A chemical ___________ can be used to show the changes that occur in a chemical reaction. In a chemical equation, the substances on the left side of the arrow are the starting substances. These substances are called ______________. The ...
... A ______________ is a well-defined example of a chemical change. A chemical ___________ can be used to show the changes that occur in a chemical reaction. In a chemical equation, the substances on the left side of the arrow are the starting substances. These substances are called ______________. The ...
Introduction to Chemical Reactions and Equations Study Guide
... ___subscripts________. These numbers tell you the ratio of __atoms______ in the compound. 3. Sometimes the names of ionic compounds include roman numerals written in parenthesis after the cation’s name. What do these numbers tell you? Why aren’t they used for all ionic compounds? The charge of the m ...
... ___subscripts________. These numbers tell you the ratio of __atoms______ in the compound. 3. Sometimes the names of ionic compounds include roman numerals written in parenthesis after the cation’s name. What do these numbers tell you? Why aren’t they used for all ionic compounds? The charge of the m ...
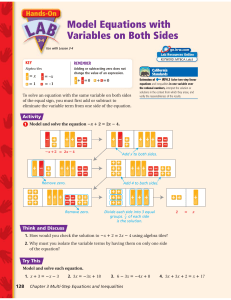
Model Equations with Variables on Both Sides
... of the equal sign, you must first add or subtract to eliminate the variable term from one side of the equation. ...
... of the equal sign, you must first add or subtract to eliminate the variable term from one side of the equation. ...
SUMMER REVIEW PACKET (for those coming into Pre
... This assignment is made up of several types of problems from the Prerequisite chapter in Pre-Calculus. This is not a required assignment but is recommended for you to be sure you are ready for Pre-Calculus next year. Reviewing this material ahead of time will help us move on to NEW content earlier a ...
... This assignment is made up of several types of problems from the Prerequisite chapter in Pre-Calculus. This is not a required assignment but is recommended for you to be sure you are ready for Pre-Calculus next year. Reviewing this material ahead of time will help us move on to NEW content earlier a ...
namimg compounds
... • When the ions come together to form compounds, they combine in a ratio that gives the compound a total charge of zero. There must be enough negative charges to balance the positive charges and vice versa. • Sodium ions have a+1 charge and chloride ions have a -1 charge. Add these charges ...
... • When the ions come together to form compounds, they combine in a ratio that gives the compound a total charge of zero. There must be enough negative charges to balance the positive charges and vice versa. • Sodium ions have a+1 charge and chloride ions have a -1 charge. Add these charges ...
Chem 30A Fa_06 FE Review
... The reaction between ammonia and carbon dioxide forms urea, CH4N2O(s), according to the following equation: 2NH3(g) + CO2(g) CH4N2O(s) + H2O(l) If 75.0 g of NH3 is reacted with 92.5 g of CO2, how many grams of urea are formed? If 115 g of urea is actually obtained, what is the percent yield? (Hint ...
... The reaction between ammonia and carbon dioxide forms urea, CH4N2O(s), according to the following equation: 2NH3(g) + CO2(g) CH4N2O(s) + H2O(l) If 75.0 g of NH3 is reacted with 92.5 g of CO2, how many grams of urea are formed? If 115 g of urea is actually obtained, what is the percent yield? (Hint ...
Math 2250-10 Quiz 2 SOLUTIONS January 17, 2014
... Since y t is differentiable it's also continuous, so y t K 4 also is - and is never zero. Thus y K 4 = CeK2 x C ...
... Since y t is differentiable it's also continuous, so y t K 4 also is - and is never zero. Thus y K 4 = CeK2 x C ...
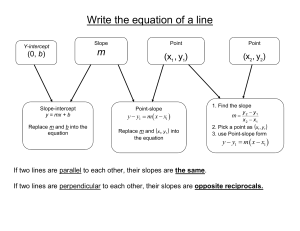
5.1 Writing Equations in Slope
... A plumber charges $66 for the first hour of a service call and $45 for any additional hours. Write a linear model to reflect the plumber’s charges and use the equation to determine the cost of a 3-hour service call. Let C represent the cost of the service and h represent the number of hours. ...
... A plumber charges $66 for the first hour of a service call and $45 for any additional hours. Write a linear model to reflect the plumber’s charges and use the equation to determine the cost of a 3-hour service call. Let C represent the cost of the service and h represent the number of hours. ...
Mon, Mar 17
... The following is a DE of a different type since it contains the dependent variable: y ' = .08y Say in words what this says! Note that we don’t see the independent variable at all – let’s call it t . What is a solution to this equation? And how can we find it? ...
... The following is a DE of a different type since it contains the dependent variable: y ' = .08y Say in words what this says! Note that we don’t see the independent variable at all – let’s call it t . What is a solution to this equation? And how can we find it? ...
Chemistry Post-Enrolment Worksheet C
... To represent a chemical reaction we could write a word or symbol equation. At A level, you will be expected to interpret, construct and balance symbol equations. ...
... To represent a chemical reaction we could write a word or symbol equation. At A level, you will be expected to interpret, construct and balance symbol equations. ...