Regulation of Glycolysis - Valdosta State University
... – Protein has regulatory site • Competition between glucose and fructose 6-phosphate – Glucose stimulates release of hexokinase IV into cytoplasm – Fructose 6-phosphate inhibits this process • Hexokinase IV not affected by glucose 6phosphate as the other isozymes are ...
... – Protein has regulatory site • Competition between glucose and fructose 6-phosphate – Glucose stimulates release of hexokinase IV into cytoplasm – Fructose 6-phosphate inhibits this process • Hexokinase IV not affected by glucose 6phosphate as the other isozymes are ...
File - Mrs Jones A
... mitochondria use oxygen; chloroplasts produce oxygen; mitochondria are always active / respiration continues independently of light; chloroplasts are inactive in dark / photosynthesis does not take place without light; oxygen released by, chloroplasts / photosynthesis, can be utilised by mitochondri ...
... mitochondria use oxygen; chloroplasts produce oxygen; mitochondria are always active / respiration continues independently of light; chloroplasts are inactive in dark / photosynthesis does not take place without light; oxygen released by, chloroplasts / photosynthesis, can be utilised by mitochondri ...
Chapter 8 – an introduction to metabolism
... 8. Describe how the carbon skeleton of glucose changes as it proceeds through glycolysis. 9. Explain why ATP is required for the preparatory steps of glycolysis. 10. Identify where substrate-level phosphorylation and the reduction of NAD+ occur in glycolysis. 11. Describe where pyruvate is oxidized ...
... 8. Describe how the carbon skeleton of glucose changes as it proceeds through glycolysis. 9. Explain why ATP is required for the preparatory steps of glycolysis. 10. Identify where substrate-level phosphorylation and the reduction of NAD+ occur in glycolysis. 11. Describe where pyruvate is oxidized ...
Document
... absorb and transfer sugars / energy. 12. The light-independent reactions require light / do not require light, and they build sugars / energy. 13. Use the space below to sketch a chloroplast. Label the grana, thylakoids, and stroma. Indicate where each of the following steps of the photosynthetic pr ...
... absorb and transfer sugars / energy. 12. The light-independent reactions require light / do not require light, and they build sugars / energy. 13. Use the space below to sketch a chloroplast. Label the grana, thylakoids, and stroma. Indicate where each of the following steps of the photosynthetic pr ...
Oxidative Phosphorylation - Study in Universal Science College
... intermembrane space Complex I – k/as proton pump driven by the energy of electron transfer; where – protons move from one location (matrix which then becomes negatively charged) to the other (intermembrane space which becomes ...
... intermembrane space Complex I – k/as proton pump driven by the energy of electron transfer; where – protons move from one location (matrix which then becomes negatively charged) to the other (intermembrane space which becomes ...
Gluconeogensis
... b. transfers CO2 to N forming molecule on the right c. Biotin is covalently bound to enzyme d. Enzyme also binds pyruvate & deprotonates it i. Adds CO2 to pyruvate resulting in oxaloacetate e. Process requires energy (ATP) f. Takes place in mitochondria!!! – very easy exam question i. Pyruvate Carbo ...
... b. transfers CO2 to N forming molecule on the right c. Biotin is covalently bound to enzyme d. Enzyme also binds pyruvate & deprotonates it i. Adds CO2 to pyruvate resulting in oxaloacetate e. Process requires energy (ATP) f. Takes place in mitochondria!!! – very easy exam question i. Pyruvate Carbo ...
McFil: metabolic carbon flow in leaves
... acids only, (ii) de novo biosynthesis plus protein assembly, which included an additional 3.5 ...
... acids only, (ii) de novo biosynthesis plus protein assembly, which included an additional 3.5 ...
Chapter 7
... NADH, FADH2, and NADPH are important carriers of hydrogen and high-energy electrons. NADH and FADH2 are used in making ATP, while NADPH is used in biosynthetic reactions. C. NADPH: An Energy Shuttle for Biosynthesis Key terms: ATP, NADH, FADH2, NADPH, biosynthesis, ADP, pyrophosphate, AMP, GTP, NAD, ...
... NADH, FADH2, and NADPH are important carriers of hydrogen and high-energy electrons. NADH and FADH2 are used in making ATP, while NADPH is used in biosynthetic reactions. C. NADPH: An Energy Shuttle for Biosynthesis Key terms: ATP, NADH, FADH2, NADPH, biosynthesis, ADP, pyrophosphate, AMP, GTP, NAD, ...
File
... Answer = A 2. Cell communication is critical for the function of both unicellular and multicellular eukaryotes. Which of the following is likely true of cell signaling? A. cell signaling uses the highest molecular weight molecules found in living cells B. Cell signaling has largely been replaced by ...
... Answer = A 2. Cell communication is critical for the function of both unicellular and multicellular eukaryotes. Which of the following is likely true of cell signaling? A. cell signaling uses the highest molecular weight molecules found in living cells B. Cell signaling has largely been replaced by ...
Photosynthesis Light-Dependent Reactions Calvin Cycle
... thylakoid space, producing ATP & NADPH in the process (Don’t worry about the details of the ETC). The ATP & NADPH are then used in the next stage, the light-independent reactions (Calvin Cycle). ...
... thylakoid space, producing ATP & NADPH in the process (Don’t worry about the details of the ETC). The ATP & NADPH are then used in the next stage, the light-independent reactions (Calvin Cycle). ...
Describe how cells are used in the production of
... (maximum of three) • Enzyme controlled reaction (only if not already awarded the mark) • Energy released (only if not already awarded the mark) • Pyruvic acid • Broken down to carbon dioxide and water • Oxygen required/aerobic • 36 ATP produced/total 38 ATP produced (per glucose molecule) ...
... (maximum of three) • Enzyme controlled reaction (only if not already awarded the mark) • Energy released (only if not already awarded the mark) • Pyruvic acid • Broken down to carbon dioxide and water • Oxygen required/aerobic • 36 ATP produced/total 38 ATP produced (per glucose molecule) ...
Muscle Metabolism - Interactive Physiology
... reticulum will act as a hydrolytic enzyme when it hydrolyzes ATP into ADP and Pi. The energy released is used to change the shape of the pump allowing the calcium ions to go back into the SR. • During the reaction, the water (shown in blue) breaks the high energy bond between the last two phosphate ...
... reticulum will act as a hydrolytic enzyme when it hydrolyzes ATP into ADP and Pi. The energy released is used to change the shape of the pump allowing the calcium ions to go back into the SR. • During the reaction, the water (shown in blue) breaks the high energy bond between the last two phosphate ...
Slide 1 - Ommbid.com
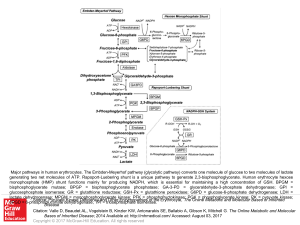
... Major pathways in human erythrocytes. The Embden-Meyerhof pathway (glycolytic pathway) converts one molecule of glucose to two molecules of lactate generating two net molecules of ATP. Rapoport-Luebering shunt is a unique pathway to generate 2,3-bisphosphoglycerate. Human erythrocyte hexose monophos ...
... Major pathways in human erythrocytes. The Embden-Meyerhof pathway (glycolytic pathway) converts one molecule of glucose to two molecules of lactate generating two net molecules of ATP. Rapoport-Luebering shunt is a unique pathway to generate 2,3-bisphosphoglycerate. Human erythrocyte hexose monophos ...
Energy Metabolism
... energy and nutrients into form that cells can use Maintenance – repairing i i body b d parts and keeping organs functioning ...
... energy and nutrients into form that cells can use Maintenance – repairing i i body b d parts and keeping organs functioning ...
Chocolate Wasted 40 Answer
... C3 plants—use CO2 in Calvin Cycle to create the 6 carbon molecule (Carbon Fixation) C4 plants—create oxaloacetate or malic acid (both 4 C molecules) as holder of carbon overnight ...
... C3 plants—use CO2 in Calvin Cycle to create the 6 carbon molecule (Carbon Fixation) C4 plants—create oxaloacetate or malic acid (both 4 C molecules) as holder of carbon overnight ...
Respiration, Lithotrophy & Photosynthesis
... In redox reactions, the DG values are proportional to the reduction potential (E) between the oxidized form (e– acceptor) and its reduce form (e– donor) - The reduction potential is a measure of the tendency of a molecule to accept electrons. A reaction is favored by positive values of E, which yiel ...
... In redox reactions, the DG values are proportional to the reduction potential (E) between the oxidized form (e– acceptor) and its reduce form (e– donor) - The reduction potential is a measure of the tendency of a molecule to accept electrons. A reaction is favored by positive values of E, which yiel ...
A report published August 2006 demonstrated that peptide YY:
... electrons will be removed) to CO2 • Oxygen is reduced (Hs will be added) to H2O • Energy is released Fall 2007, Bio 93, O’Dowd and Warrior, UCI - Copyright: All rights reserved ...
... electrons will be removed) to CO2 • Oxygen is reduced (Hs will be added) to H2O • Energy is released Fall 2007, Bio 93, O’Dowd and Warrior, UCI - Copyright: All rights reserved ...
Monday Oct
... • Slow twitch = slower hydrolysis, isozyme catalyzes the reaction slower Myosin isozymes not modified by athletic training! ...
... • Slow twitch = slower hydrolysis, isozyme catalyzes the reaction slower Myosin isozymes not modified by athletic training! ...
MS Word Version - Interactive Physiology
... 8. It is called "dehydration" because water is removed during the reaction. It's called "synthesis" because a bigger molecule is synthesized from two smaller ones. 9. When muscle cells hydrolyze ATP, energy is released and, like "currency", can be "spent" for moving myofilaments and transporting ion ...
... 8. It is called "dehydration" because water is removed during the reaction. It's called "synthesis" because a bigger molecule is synthesized from two smaller ones. 9. When muscle cells hydrolyze ATP, energy is released and, like "currency", can be "spent" for moving myofilaments and transporting ion ...
Glycolysis
... • Three steps of glycolysis are irreversible and therefore need bypass reactions for gluconeogenesis. • Pyruvate to PEP: Pyruvate synthesized by glycolysis or from aa is in the mitochondria. Here, pyruvate is first converted to oxaloacetate by the enzyme pyruvate carboxylase. One carbon is supplied ...
... • Three steps of glycolysis are irreversible and therefore need bypass reactions for gluconeogenesis. • Pyruvate to PEP: Pyruvate synthesized by glycolysis or from aa is in the mitochondria. Here, pyruvate is first converted to oxaloacetate by the enzyme pyruvate carboxylase. One carbon is supplied ...
Document
... p = the fraction of the population with CF chromosomes (~ 0.05) ( p << 1; it’s hard to imagine a mechanism that would confer a great selective advantage to the heterozygote while remaining lethal for the homozygote). ...
... p = the fraction of the population with CF chromosomes (~ 0.05) ( p << 1; it’s hard to imagine a mechanism that would confer a great selective advantage to the heterozygote while remaining lethal for the homozygote). ...
Chapter 9 powerpoint - Red Hook Central Schools
... • The process in cell respiration that generates most of the ATP is oxidative phosphorylation (powered by redox reactions). • Oxidative phosphorylation accounts for almost 90% of the ATP generated by cellular respiration. • A smaller amount of ATP is formed in glycolysis and the citric acid / Krebs ...
... • The process in cell respiration that generates most of the ATP is oxidative phosphorylation (powered by redox reactions). • Oxidative phosphorylation accounts for almost 90% of the ATP generated by cellular respiration. • A smaller amount of ATP is formed in glycolysis and the citric acid / Krebs ...
State a significant event that occurs during each of the following
... Question 12: Briefly describe (not list) two mechanisms during meiosis that create genetic variation. (6 pts) Synapsis & crossing over – creates unique new chromosomes consisting of a mixture of paternal and maternal genes Independent assortment of homologous pairs – creates unique combinations of m ...
... Question 12: Briefly describe (not list) two mechanisms during meiosis that create genetic variation. (6 pts) Synapsis & crossing over – creates unique new chromosomes consisting of a mixture of paternal and maternal genes Independent assortment of homologous pairs – creates unique combinations of m ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.