Chapter 5 Test Review
... 6. enter the electron transport chain of the thylakoid membrane 7. water is split to replace 2e- that enter the electron transport chain of the thylakoid membrane, the O2 is released and the H+ join with NADP+ 8. PSII – ATP, PSI – NADPH 9. ATP (to Calvin cycle), NADPH (to Calvin Cycle), O2 (released ...
... 6. enter the electron transport chain of the thylakoid membrane 7. water is split to replace 2e- that enter the electron transport chain of the thylakoid membrane, the O2 is released and the H+ join with NADP+ 8. PSII – ATP, PSI – NADPH 9. ATP (to Calvin cycle), NADPH (to Calvin Cycle), O2 (released ...
Cell Respiration State that oxidation involves the loss of electrons
... with oxygen and form water. Energy is released during the process, which is controlled and used by the cell in the form of ATP. For each molecules of NAD that is oxidised, 3 molecules of ATP are formed. In total, aerobic respiration forms 38 moIecuIes of ATP for each molecule of glucose. ...
... with oxygen and form water. Energy is released during the process, which is controlled and used by the cell in the form of ATP. For each molecules of NAD that is oxidised, 3 molecules of ATP are formed. In total, aerobic respiration forms 38 moIecuIes of ATP for each molecule of glucose. ...
Chapter 4 Cellular Metabolism
... where protein synthesis will occur. They lie across the __ribosome_ and wait for the ___transfer RNA to bring in the appropriate amino acids. The correct amino acids will be lined up because the tRNA bases are arranged in _anti-codons_ that are complementary to the __cocons_ of the bases of the mRNA ...
... where protein synthesis will occur. They lie across the __ribosome_ and wait for the ___transfer RNA to bring in the appropriate amino acids. The correct amino acids will be lined up because the tRNA bases are arranged in _anti-codons_ that are complementary to the __cocons_ of the bases of the mRNA ...
Answer Set 2
... b. 5 mCi/mmol. The specific activity is halved because the number of moles of product (pyruvate) is twice that of the labeled substrate (glucose). GG18-3. Increased [ATP], [citrate], or [glucose-6-phosphate] inhibits glycolysis. Increased [AMP], [fructose-1,6-bisphosphate], and [fructose-2,6-bisphos ...
... b. 5 mCi/mmol. The specific activity is halved because the number of moles of product (pyruvate) is twice that of the labeled substrate (glucose). GG18-3. Increased [ATP], [citrate], or [glucose-6-phosphate] inhibits glycolysis. Increased [AMP], [fructose-1,6-bisphosphate], and [fructose-2,6-bisphos ...
Cell Respiration Cellular Respiration Aerobic Respiration Aerobic
... • Electrons from NADH and FADH2 are transported along an electron transport chain • Energy released used to produce ATP ...
... • Electrons from NADH and FADH2 are transported along an electron transport chain • Energy released used to produce ATP ...
PowerPoint
... • 3 CO2, 1 GTP, 4 NADH and 1 FADH2 produced for each pyruvate molecule. • Total: 6CO2, 2 GTP, 8 NADH, 2FADH2 ...
... • 3 CO2, 1 GTP, 4 NADH and 1 FADH2 produced for each pyruvate molecule. • Total: 6CO2, 2 GTP, 8 NADH, 2FADH2 ...
External sources of energy → biologically energy : ATP
... • Electron transport chain • High energy electrons from NADH and FADH2 O2 • Convert energy released into a proton motive force (H+ gradient) ...
... • Electron transport chain • High energy electrons from NADH and FADH2 O2 • Convert energy released into a proton motive force (H+ gradient) ...
File
... H+ ions and oxygen (final electron acceptor) to form water Creates a concentration gradient of H+ ions = proton-motive ...
... H+ ions and oxygen (final electron acceptor) to form water Creates a concentration gradient of H+ ions = proton-motive ...
Document
... Overview of glycolysis plus the citric acid cycle plus transfer of energy from reduced carriers (NADH, FADH2) to ATP via the electron transport system, which involves a series of proteins that can carry out the energy transfer reactions. Note the role of atmospheric oxygen in this! ...
... Overview of glycolysis plus the citric acid cycle plus transfer of energy from reduced carriers (NADH, FADH2) to ATP via the electron transport system, which involves a series of proteins that can carry out the energy transfer reactions. Note the role of atmospheric oxygen in this! ...
Biochemistry I, Spring Term 2000 - Third Exam
... B3 (6 pts): *The membrane potential across the mitochondrial membrane is approximately -150 mV (inside negative). The oxidation of each NADH pumps about 8 protons across this membrane. This generates a pH difference of 1 pH unit and stores about 160 kJ/mol of free energy (i.e. 20 kJ/mol per proton). ...
... B3 (6 pts): *The membrane potential across the mitochondrial membrane is approximately -150 mV (inside negative). The oxidation of each NADH pumps about 8 protons across this membrane. This generates a pH difference of 1 pH unit and stores about 160 kJ/mol of free energy (i.e. 20 kJ/mol per proton). ...
Exam I Review - Iowa State University
... water into the matrix through the ATP synthase channel by osmosis, and the energy in this water flow is used to power ATP synthesis. *c. H+ movement down a concentration gradient from the intermembrane space into the mitochondrial matrix through ATP synthase results in ATP synthesis d. All these sta ...
... water into the matrix through the ATP synthase channel by osmosis, and the energy in this water flow is used to power ATP synthesis. *c. H+ movement down a concentration gradient from the intermembrane space into the mitochondrial matrix through ATP synthase results in ATP synthesis d. All these sta ...
Chapter 1 - TeacherWeb
... Cellular respiration – name four phases, starting reactants/ending products of each phase, location of each process, general understanding of each process, number of ATP & product at each stage produced by 1 glucose molecule Role of NAD+, FAD, Coenzyme A Similarities and differences between aerobic ...
... Cellular respiration – name four phases, starting reactants/ending products of each phase, location of each process, general understanding of each process, number of ATP & product at each stage produced by 1 glucose molecule Role of NAD+, FAD, Coenzyme A Similarities and differences between aerobic ...
Quiz 6
... 4. Thrown out 5. When ATP releases energy, it also releases inorganic phosphate. What purpose does this serve (if any) in the cell? A) It is released as an excretory waste. B) It is used exclusively to regenerate more ATP. C) It can be added to water and excreted as a liquid. D) It can be added to o ...
... 4. Thrown out 5. When ATP releases energy, it also releases inorganic phosphate. What purpose does this serve (if any) in the cell? A) It is released as an excretory waste. B) It is used exclusively to regenerate more ATP. C) It can be added to water and excreted as a liquid. D) It can be added to o ...
Slide 1
... Cellular respiration makes ATP by breaking down sugars. If a step requires oxygen, it is called aerobic. If a step occurs in the absence of oxygen, it is called anaerobic. It takes place in three steps: Glycolysis Krebs cycle Electron transport chain ...
... Cellular respiration makes ATP by breaking down sugars. If a step requires oxygen, it is called aerobic. If a step occurs in the absence of oxygen, it is called anaerobic. It takes place in three steps: Glycolysis Krebs cycle Electron transport chain ...
ch3b FA11 - Cal State LA
... • Redox reactions: the gain (reduction) or loss (oxidation) of electrons – Changes in organic molecules shift the degree of e- sharing • Carbon in C-H bond is reduced • Carbon in C=O bond is oxidized – EN diffs result in e- spending less time around C when bonded to O ...
... • Redox reactions: the gain (reduction) or loss (oxidation) of electrons – Changes in organic molecules shift the degree of e- sharing • Carbon in C-H bond is reduced • Carbon in C=O bond is oxidized – EN diffs result in e- spending less time around C when bonded to O ...
Lecture 17/18 - Aerobic and Anaerobic Metabolism
... 4.) What is the energy yield of the citric acid cycle? What type of phosphorylation produces the GTP? 5.) Summarize the overall net energy yield for 1 molecule of glucose that undergoes glycolysis and the CAC—It may be helpful to include the energy reactants and products 6.) Explain how the concent ...
... 4.) What is the energy yield of the citric acid cycle? What type of phosphorylation produces the GTP? 5.) Summarize the overall net energy yield for 1 molecule of glucose that undergoes glycolysis and the CAC—It may be helpful to include the energy reactants and products 6.) Explain how the concent ...
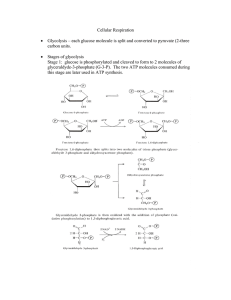
Cellular Respiration - Seattle Central College
... glucose and the conversion of Fructose-6-phosphate to Fructose-1,6-diphosphate. The net production of ATP per glucose is 2. ...
... glucose and the conversion of Fructose-6-phosphate to Fructose-1,6-diphosphate. The net production of ATP per glucose is 2. ...
Light Independent
... cytoplasm ● Does NOT require oxygen ● Net gain = 2 ATP One day you will learn that this is a complicated process, but for now… big picture! ...
... cytoplasm ● Does NOT require oxygen ● Net gain = 2 ATP One day you will learn that this is a complicated process, but for now… big picture! ...
File
... 1. What is the energy molecule of the cell called? ATP 2. What macromolecule made by plants is "burned" in the mitochondria? GLUCOSE 3. Where is chlorophyll found in the chloroplast? THYLAKOIDS 4. In which part of a plant would you expect to find the most chloroplasts and why? LEAVES – HAVE THE GREA ...
... 1. What is the energy molecule of the cell called? ATP 2. What macromolecule made by plants is "burned" in the mitochondria? GLUCOSE 3. Where is chlorophyll found in the chloroplast? THYLAKOIDS 4. In which part of a plant would you expect to find the most chloroplasts and why? LEAVES – HAVE THE GREA ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.